You are using an outdated browser. Upgrade your browser today or install Google Chrome Frame to better experience this site.
- Section 5 - Yellow Fever
- Section 5 - Amebiasis
CDC Yellow Book 2024
Author(s): Stacey Martin, J. Erin Staples
Infectious Agent
Transmission, epidemiology, clinical presentation.
INFECTIOUS AGENT: Zika virus
Worldwide, periodic outbreaks in tropical and subtropical regions
TRAVELER CATEGORIES AT GREATEST RISK FOR EXPOSURE & INFECTION
PREVENTION METHODS
Avoid insect bites
Use condoms or abstain from sex if exposed (or possibly exposed)
DIAGNOSTIC SUPPORT
Zika virus is a single-stranded RNA virus of the Flaviviridae family, genus Flavivirus .
Transmission occurs through the bite of an infected Aedes species mosquito. Intrauterine, perinatal, sexual, laboratory, and possible transfusion-associated transmission have been reported. Zika virus has been detected in breast milk, but the risk for transmission through breastfeeding is unknown.
Zika virus occurs in tropical and subtropical regions. Since 2007, outbreaks of Zika virus disease have occurred throughout the Pacific Islands and in Southeast Asia. In 2015, Zika virus was identified in the Western Hemisphere, where large outbreaks occurred in Brazil. The virus then spread throughout much of the Americas, resulting in several hundred thousand cases. Since 2017, the number of reported Zika virus disease cases has declined worldwide, but occasional increases in cases have been noted from some countries. In 2020, only 4 Zika virus cases were reported in US international travelers. See current information on Zika virus transmission and travel guidance .
Most Zika virus infections are either asymptomatic or result in mild clinical illness characterized by acute onset of fever, arthralgia, nonpurulent conjunctivitis, and maculopapular rash. Other symptoms can include edema, headache, lymphadenopathy, myalgia, retro-orbital pain, and vomiting. Severe disease requiring hospitalization and death are both uncommon. Guillain-Barré syndrome and rare reports of encephalopathy, meningoencephalitis, myelitis, uveitis, and severe thrombocytopenia have been associated with Zika virus infection, however. Vertical transmission of the virus leads to congenital Zika virus infection; sequelae include microcephaly with brain anomalies and other serious neurologic consequences, and fetal loss.
Consider Zika virus infection in patients with acute onset of fever, arthralgia, conjunctivitis, or maculopapular rash who, ≤2 weeks of illness onset, lived in or recently traveled to areas with ongoing Zika virus transmission or had sex with someone who lives in or traveled to those areas. Because Zika and dengue virus infections have similar clinical presentations, patients with suspected Zika virus infection also should be evaluated for possible dengue. Other considerations in the differential diagnosis include adenovirus, chikungunya, enterovirus, leptospirosis, malaria, measles, parvovirus, rickettsiosis, rubella, and group A streptococcal infections (see disease-specific chapters in this section).
Zika virus disease is a nationally notifiable condition. Report suspected cases of Zika virus infection to state or local health departments to facilitate diagnosis and mitigate the risk for local transmission in areas where Aedes species mosquitoes are active. State health departments should report laboratory-confirmed cases to the Centers for Disease Control and Prevention (CDC) according to the Council of State and Territorial Epidemiologists case definitions .
Diagnostic Testing
Because Zika and dengue viruses share a similar global geographic distribution and cause infections that can be difficult to differentiate diagnostically, consider the global epidemiology of these 2 arboviruses when requesting testing and interpreting results. Zika virus testing guidance is updated as needed to address changes in the epidemiology. Current testing guidance is provided on the CDC website. Some state health departments and many commercial laboratories perform Zika virus nucleic acid amplification testing (NAAT) and IgM testing. Confirmatory neutralizing antibody testing is available at CDC’s Arboviral Diagnostic Reference Laboratory and selected health department laboratories.
Nucleic Acid Amplification Testing
NAAT is used to detect Zika viral RNA early in the course of infection and can be performed on amniotic fluid, whole blood, cerebrospinal fluid, semen, serum, tissues, and urine. Due to the temporal nature of Zika virus RNA in the body, a negative NAAT does not always exclude recent Zika virus infection. For this reason, Zika virus IgM antibody testing might be recommended in certain situations.
Serologic Testing
Serum IgM antibody testing can detect Zika virus–specific IgM antibodies that typically develop toward the end of the first week of illness and can remain detectable for months to years after infection, making the determination of the timing of infection difficult. Serum IgM antibody testing can result in a false-positive result due to cross-reacting antibodies against related flaviviruses (e.g., dengue virus, yellow fever virus). Plaque reduction neutralization testing (PRNT) can be used to discriminate between cross-reacting antibodies in primary flavivirus infections, but neutralizing antibodies might still yield cross-reactive results in people who were previously infected with or vaccinated against a related flavivirus (secondary flavivirus infection).
No specific antiviral treatment is available for Zika virus disease. Treatment is generally supportive and can include use of analgesics and antipyretics, fluids, and rest. Because aspirin and other nonsteroidal anti-inflammatory drugs (NSAIDs) can increase the risk for hemorrhage in patients with dengue, avoid use of these medications until dengue can be ruled out.
Protect people infected with Zika, chikungunya, or dengue virus from further mosquito exposure during the first week of illness to decrease the possibility of local transmission. Carefully evaluate pregnant people with laboratory evidence of Zika virus infection; closely manage these cases for possible adverse pregnancy outcomes. See guidance for the diagnosis, evaluation, and management of infants with possible congenital Zika virus infections.
No vaccine or preventive drug is available for Zika virus. All travelers to areas with Zika virus transmission should take steps to avoid mosquito bites to prevent Zika virus and other vectorborne infections (see Sec. 4, Ch. 6, Mosquitoes, Ticks & Other Arthropods ). Advise people with possible Zika virus exposure who want to reduce the risk for sexual transmission of Zika virus to an uninfected partner to follow current CDC recommendations . Although blood donations in the United States were previously screened for Zika virus RNA, the US Food and Drug Administration ceased this requirement in May 2021 because the virus no longer has sufficient incidence to affect the potential donor population.
Pregnant people should not travel to areas with ongoing Zika outbreaks . Before traveling to areas with current or past spread of Zika, pregnant people should discuss their travel plans with a health care provider. In deciding whether to travel, pregnant people should consider the destination, their reasons for traveling, and their ability to prevent mosquito bites. If used in accordance with the instructions on the product label, there are no restrictions on the use of insect repellents by people who are pregnant.
If a pregnant person or their partner travels to an area with current or past spread of Zika virus, advise the couple to use condoms or to abstain from sex for the entire pregnancy, even if the traveler does not have symptoms of Zika or feel sick. Couples trying to become pregnant who travel to areas with past or current Zika virus transmission should take steps to protect themselves from Zika and consider waiting to get pregnant according to the timeframes outlined in CDC guidance .
Mothers are encouraged to breastfeed infants even after possible Zika virus exposure, because available evidence indicates the benefits of breastfeeding outweigh the theoretical risks associated with Zika virus infection transmission through breast milk.
CDC website: Zika
The following authors contributed to the previous version of this chapter: J. Erin Staples, Stacey W. Martin, Marc Fischer
Bibliography
Adebanjo T, Godfred-Cato S, Viens L, Fischer M, Staples JE, Kuhnert-Tallman W, et al. Update: interim guidance for the diagnosis, evaluation, and management of infants with possible congenital Zika virus infection—United States, October 2017. MMWR Morb Mortal Wkly Rep. 2017;66(41):1089–99.
Angelo KM, Stoney RJ, Brun-Cottan G, Leder K, Grobusch MP, Hochberg N, et al. Zika among international travellers presenting to GeoSentinel sites, 2012–2019: implications for clinical practice. J Travel Med. 2020;27(4): taaa061.
Gregory CJ, Oduyebo T, Brault AC, Brooks JT, Chung KW, Hills S, et al. Modes of transmission of Zika virus. J Infect Dis. 2017;216(10):S875–83.
Hills SL, Fischer M, Petersen LR. Epidemiology of Zika virus infection. J Infect Disease. 2017;216(10):S868–74.
Oduyebo T, Polen KD, Walke HT, Reagan-Steiner S, Lathrop E, Rabe IB, et al. Update: interim guidance for health care providers caring for pregnant women with possible Zika virus exposure—United States (including U.S. territories), July 2017. MMWR Morb Mortal Wkly Rep. 2017;66(29):781–93.
Sharp TM, Fischer M, Munoz-Jordan JL, Paz-Bailey G, Staples JE, Gregory CJ, et al. Dengue and Zika virus diagnostic testing for patients with a clinically compatible illness and risk for infection with both viruses. MMWR Recomm Rep.
File Formats Help:
- Adobe PDF file
- Microsoft PowerPoint file
- Microsoft Word file
- Microsoft Excel file
- Audio/Video file
- Apple Quicktime file
- RealPlayer file
- Zip Archive file
- Type 2 Diabetes
- Heart Disease
- Digestive Health
- Multiple Sclerosis
- Diet & Nutrition
- Health Insurance
- Public Health
- Patient Rights
- Caregivers & Loved Ones
- End of Life Concerns
- Health News
- Thyroid Test Analyzer
- Doctor Discussion Guides
- Hemoglobin A1c Test Analyzer
- Lipid Test Analyzer
- Complete Blood Count (CBC) Analyzer
- What to Buy
- Editorial Process
- Meet Our Medical Expert Board
Zika Virus Facts and Statistics: What You Need to Know
- How Common Is Zika Virus?
- Age & Gender
- Causes and Risk Factors
- Mortality Rates
Screening and Early Detection
- Next in Zika Guide What Is Zika Virus?
The most recent Zika virus outbreak in the United States (U.S.) occurred in 2016, and 5,168 people were diagnosed with Zika that year. Since 2018, Zika cases have been on the decline, and as of July 2022, there are no current outbreaks of Zika worldwide. This article will highlight important facts and statistics about the Zika virus.
Orbon Alija / Getty Images
Zika Virus Overview
Zika is a rare, mosquito-borne illness caused by the Flaviviridae virus. If a Zika-infected mosquito bites the human skin, the virus can travel into that person's bloodstream. Zika can also pass to a fetus through the placenta during pregnancy. Less commonly, it can also be transmitted through sex and blood.
Most people with Zika don’t show any symptoms . When symptoms occur, they are usually mild and include:
- Joint or muscle pain
- Pink eye ( conjunctivitis )
- Swollen lymph nodes ( lymphadenopathy )
Zika can lead to complications, including:
- Congenital disorders
- Guillain-Barré syndrome (an autoimmune disorder)
- Increased risk of stillbirth during pregnancy and spontaneous pregnancy loss
How Common Is Zika Virus?
The first Zika virus outbreak in the U.S. was in 2016, and cases spiked that year. However, cases have been on the decline since 2018.
In 2022, getting the Zika virus in the U.S. is very rare because there is no current outbreak. The following are Zika statistics from the United States and its territories over the past several years:
- Of the 224 locally acquired cases in 2016, 218 were contracted in Florida, and six were contracted in Texas.
- There have never been reported cases in Alaska or Hawaii.
- The last reported locally acquired cases in the continental U.S. were in 2017.
- The last confirmed case in the U.S. territories occurred in 2019.
- There have been 32 documented travel-associated cases since 2019.
The following table provides facts from available statistics from 2015–2020 in the U.S. and U.S. territories.
The locally acquired cases of Zika reported in the U.S. Territories in 2020 were discovered by antibody testing. The test cannot differentiate an active virus from an old infection, so the number does not represent the number of active Zika virus cases that year.
Zika Virus by Age and Gender
Previous reports show that the Zika infection rate is higher among people ages 20–49 and among people assigned female at birth. The following are statistics regarding the 5,168 confirmed Zika cases in the U.S. in 2016:
- About 80% were 20–59 years old.
- The average age was 37.
- People assigned female at birth accounted for 3,307 (64%) of the cases.
- The majority of cases (95%) were related to travel.
- Forty-five cases were sexually transmitted; of those, 43 (96%) were people assigned female at birth.
These reports note that the sex differences in confirmed cases could be due to increased testing. The possibility of experiencing pregnancy complications and passing on congenital disorders influenced many non-menopausal adults to test.
Causes of Zika Virus and Risk Factors
Flaviviridae, a virus that an Aedes aegypti mosquito can carry, causes Zika. The Aedes mosquito thrives in wet areas such as the Gulf Coast of the U.S., especially in the warmer months. The risk is highest when the Aedes mosquito is most active; two hours after sunrise and a few hours before sunset.
Zika can spread through:
- Mosquito bites (most common)
- Pregnancy (from mother or parent to fetus)
- Barrier-free sex (penis-to-vagina transmission is more likely)
- Sharing blood (e.g., through needles)
Pregnancy and Zika Virus
Though pregnancy does not increase the risk of getting Zika, getting Zika while pregnant increases the risk of pregnancy complications and congenital disorders.
Risk factors for getting the Zika virus include:
- Traveling or living in a warm, wet climate
- Having unprotected (condomless) sex with an infected partner
- Blood transfusions
What Are the Mortality Rates for Zika Virus?
The survival rate is the percentage of people who survive a disease for a specified time, but it may be presented in several different ways.
The overall survival rate for non-congenital (adult) Zika is over 99%. Severe illness or death due to Zika is extremely rare. The following data is from the 5,168 U.S. Zika cases in 2016:
- There were 153 (3%) people hospitalized.
- There were 15 (0.3%) people diagnosed with Guillain-Barré syndrome.
- There was one reported death.
What Do We Know About the One Reported Death?
A case study shows that the one reported death occurred in a 73-year-old man. He had a decreased immune system from radiation therapy, which he completed for prostate cancer a month before getting travel-related Zika.
The Zika virus can cause pregnancy complications and congenital disorders related to the eye or brain. U.S. Zika 2016 outbreak statistics related to pregnancy or babies include:
- About 5% of babies with birthing parents who had Zika during pregnancy were born with Zika-associated conditions present at birth.
- The highest number of congenital disorders occurred six months after the outbreak's peak.
- Areas with widespread local transmission had four times the number of congenital disorders.
- Zika-associated congenital disorders occurred with two out of 25 (8%) infections in the first trimester , 6% in the second trimester, and 3.8% in the third trimester.
Because the number of Zika cases is currently low, very few people require Zika testing. When needed, diagnosis occurs through the following blood tests:
- Molecular : A molecular test detects a current Zika infection.
- Serological : The serological test looks for antibodies. It is not recommended for pregnant people because it can detect antibodies from past infections or similar viruses.
Testing is recommended if you:
- Have symptoms of Zika, even if you feel better
- Traveled to a country with a current Zika outbreak
- Are pregnant and have Zika-associated fetal anomalies on an ultrasound
- Have delivered a baby with a congenital disorder that could be related to Zika
- Are pregnant and were recently exposed
Prevention Techniques
There is currently no vaccine or medication available to prevent the Zika virus. However, when you are in high-risk areas or seasons, you can follow these Zika prevention techniques:
- Avoid areas with an outbreak while pregnant.
- Have sex with barrier devices such as condoms or avoid sex, especially while pregnant.
- Remove standing water around your home.
- Stay indoors when possible.
- Use an Environmental Protection Agency-registered insect repellent when outdoors.
- Wear long-sleeve shirts or pants when outdoors.
Anyone who travels to high-risk areas should take steps to prevent mosquito bites for three weeks upon returning home. They should also practice safer sex for several months because Zika can stay in semen for months after infection.
Learn More About Current Outbreaks
You can learn about current outbreaks through the updated Centers for Disease Control and Prevention (CDC) list . Before traveling, search by disease or country and follow these color-coded alerts:
- Green or level 1 : Low concern, take usual precautions
- Orange or level 2 : Practice additional precautions, especially for at-risk populations
- Red or level 3 : Avoid non-essential travel to that area
As of July 2022, there are no current outbreaks of Zika worldwide. However, the most current significant outbreak was in India in November of 2021.
The following are regions that have had outbreaks in the past:
- Pacific Islands
- South and Central America
- Southeast Asia
- The Caribbean
- U.S. Gulf Coast
Zika is a rare virus most commonly spread to humans through bites from the Aedes mosquito. You can also spread it through sex and infected blood or to a fetus during pregnancy. Zika can cause pregnancy complications and congenital disorders.
There are no medicines or vaccines to help prevent Zika, so other prevention techniques are important, especially for pregnant people. Those who travel can bring the virus back with them and should use prevention techniques after they return home.
Centers for Disease Control and Prevention (CDC). Statistics and maps .
World Population Review. Zika virus countries 2022 .
Centers for Disease Control and Prevention (CDC). Zika virus: What you need to know .
World Health Organization. Zika virus .
Centers for Disease Control and Prevention (CDC). Zika and pregnancy .
Johns Hopkins Medicine. What is Zika virus?
World Health Organization. Countries with current or previous Zika virus transmission .
Centers for Disease Control and Prevention (CDC). Travel health notices .
Hall V, Walker WL, Lindsey NP, et al. Update: Noncongenital Zika virus disease cases — 50 U.S. States and the District of Columbia, 2016. MMWR Morb Mortal Wkly Rep. 2018;67:265–269. doi: 10.15585/mmwr.mm6709a1
Centers for Disease Control and Prevention. Zika cases in the United States .
Centers for Disease Control and Prevention (CDC). Dengue and the Aedes aegypti mosquito .
Centers for Disease Control and Prevention (CDC). Zika virus .
Paz-Bailey G, Rosenberg ES, Doyle K, et al. Persistence of Zika virus in body fluids — Final report . N Engl J Med . 2017;379(13):1234-1243. doi:10.1056/NEJMoa1613108
Centers for Disease Control and Prevention (CDC). Zika transmission .
Swaminathan S, Schlaberg R, Lewis J, et al. Fatal Zika virus infection with secondary nonsexual transmission . N Engl J Med . 2016;375:1907-1909. doi: 10.1056/NEJMc1610613
Centers for Disease Control and Prevention (CDC). Testing for zika .
Centers for Disease Control and Prevention (CDC). Zika travel information .
By Brandi Jones, MSN-ED RN-BC Jones is a registered nurse and freelance health writer with more than two decades of healthcare experience.
Advertiser Disclosure
Many of the credit card offers that appear on this site are from credit card companies from which we receive financial compensation. This compensation may impact how and where products appear on this site (including, for example, the order in which they appear). However, the credit card information that we publish has been written and evaluated by experts who know these products inside out. We only recommend products we either use ourselves or endorse. This site does not include all credit card companies or all available credit card offers that are on the market. See our advertising policy here where we list advertisers that we work with, and how we make money. You can also review our credit card rating methodology .
Zika Virus: What It Is, Affected Countries, Symptoms, Treatment and More [Guide]
Christy Rodriguez
Travel & Finance Content Contributor
88 Published Articles
Countries Visited: 36 U.S. States Visited: 31
Keri Stooksbury
Editor-in-Chief
41 Published Articles 3375 Edited Articles
Countries Visited: 50 U.S. States Visited: 28
Director of Operations & Compliance
6 Published Articles 1201 Edited Articles
Countries Visited: 10 U.S. States Visited: 20
![zika virus travel 2022 Zika Virus: What It Is, Affected Countries, Symptoms, Treatment and More [Guide]](https://upgradedpoints.com/wp-content/uploads/2019/07/Zika-Virus-e1563052065826.jpg?auto=webp&disable=upscale&width=1200)
Table of Contents
What is the zika virus, how is zika transmitted, a brief history of zika, countries with zika in 2020, zika prevention, symptoms of zika, what to do if you think you have zika, zika treatment, zika and pregnancy, zika treatment for babies, zika-free destinations, final thoughts.
We may be compensated when you click on product links, such as credit cards, from one or more of our advertising partners. Terms apply to the offers below. See our Advertising Policy for more about our partners, how we make money, and our rating methodology. Opinions and recommendations are ours alone.
The Zika virus dominated headlines in 2016 when some infected women had babies with significant birth defects. At the time, the threat of the mosquito-borne virus caused major concern, leading many travelers to change their travel plans.
Since Zika is no longer front-page news, people might think the virus is no longer a threat. However, there are still hot spots, particularly in tropical climates, that travelers should be aware of.
As you plan your travels for this summer and beyond, you might wonder how to prepare for travel to Zika-prone countries and what has become of the virus in the past few years. Here is a guide on everything you need to know about the Zika virus when traveling.
Zika is a flavivirus. There is currently no vaccine for Zika, but it is not usually a life-threatening condition. In fact, the vast majority of people with Zika have no symptoms at all.
However, there are 2 uncommon, but severe, complications that are important to note. If you have contracted Zika, you have a significantly increased chance of having these issues:
- Infected pregnant women may transmit Zika to their babies, resulting in a number of serious birth defects that are directly linked to Zika.
- The infected person has an increased risk of having Guillain-Barre syndrome.
Because of this, it is important to be knowledgeable about how and where you are most likely to contract it.
A flavivirus is transmitted primarily by ticks and mosquitoes. It can be passed along to humans in the following ways:
- Zika is spread mostly by the bite of an infected mosquito.
- Zika can be passed from a pregnant woman to her fetus . Infection during pregnancy can cause certain birth defects. Make sure to check out the section dedicated to Zika-related pregnancy concerns below.
- Zika can be sexually transmitted from an infected man or woman. This can happen months after the initial transmission.
- Though a blood transfusion (though unconfirmed in the U.S.).
Bottom Line: According to the Center for Disease Control (or CDC), there is no evidence that Zika can be transmitted through saliva [ 1 ]. There is also no evidence that Zika is transmitted through animals other than mosquitoes [ 2 ].
Zika is a concern in many parts of the world, however, since the virus is primarily transmitted through mosquitoes, this means that the greatest risk for contraction is in climates that are most favorable to mosquitoes.
Specifically, though, Zika is transmitted by the Aedes species of mosquito. These are the same mosquitoes that spread dengue and chikungunya viruses. These thrive in the Caribbean, Central and South America, Southeast Asia, and parts of Central Africa.
The Zika virus was first discovered in 1947 and is named after the Zika Forest in Uganda. In 1952, the first human cases of Zika were detected.
Before 2007, 14 cases of Zika had been documented, although other cases were likely to have occurred and were not reported. Because the symptoms of Zika are generally mild and similar to those of many other diseases, cases may not have been recognized at the time.
The first large outbreak of Zika was reported in 2007 from the Island of Yap, which is located in the Federated States of Micronesia. Since then, outbreaks of Zika have been reported in tropical Africa, Southeast Asia, and the Pacific Islands.
Zika became international news in the summer of 2016 when a massive outbreak struck South and Central America and the Caribbean. causing more than half a million suspected cases and more than 3,700 congenital birth defects, according to the World Health Organization, (or WHO) [ 3 ].
In February 2016, WHO declared that the number of cases of the Zika infection constituted a “Public Health Emergency of International Concern.”

Declining Cases
It should be noted that in most countries, cases of Zika are significantly down from the peak in 2016. In fact, in November 2016, WHO declared the end of this emergency. Ultimately, the exact decline is hard to measure since many people with no symptoms were never tested for Zika.
Most experts say the decline in Zika cases is due, at least in part, to herd immunity. This means that when enough people become immune to a virus, that disease can’t easily travel from person to person. This is especially true for individuals living in areas with a high concentration of mosquito-based infections.
There is also suspected “cross-protection” because Zika virus shares antibodies with dengue virus and other flaviviruses, but this has not been proven.
According to WHO, the Zika virus is present in more than 87 countries. As of 2020, virus activity continues in the Caribbean, most of Latin America, Central Africa, India, Indonesia, Malaysia, Cambodia, and Papua New Guinea, among other places.
Below is some information regarding the number of Zika cases over recent years in specific countries. This information is what has been reported to the European Center for Disease Prevention and Control [ 4 ] as of October 2019 and risk information from gov.uk as of July 2019 [ 5 ].
U.S. & U.S. Territories
Zika does not easily spread in the continental U.S. because Aedes mosquitoes are relatively rare, however, U.S. territories have experienced a significant number of Zika cases.
In 2016, Puerto Rico, the U.S. Virgin Islands, and American Samoa saw more than 36,000 cases of locally-transmitted Zika virus [ 6 ]. Texas and Florida saw a little over 200 cases locally-transmitted.
Since the high point in 2016, Zika in the U.S. has declined sharply and has all but disappeared. During 2019, only 57 cases were acquired through local mosquito-borne transmission in U.S. territories, and 0 cases were noted locally in the continental U.S., including Florida and Texas that had experienced cases before [ 7 ]. Alaska and Hawaii also reported no cases.
This doesn’t mean that people living in the U.S. don’t have the Zika virus, but rather that it wasn’t transmitted locally. They would have contracted the virus during their international travels.
For the most current information, the CDC has a county by county breakdown of all mosquito-borne cases reported in the U.S. by year, including Zika virus.
Below are the U.S. States and Territories that have a current risk of Zika:
Mexico & Central America
In 2019, Mexico reported 50 confirmed cases of Zika compared to 39 for the same period in 2018. This is a significant decrease in cases compared to 2018 when 860 confirmed cases were reported.
In 2018, Guatemala was one of the countries in the region reporting the highest number of cases. The good news is that only 10 cases were reported in 2019!
Below are the countries in Central America that have a current risk of Zika:
Caribbean Islands
In 2018, according to the Pan American Health Organization (PAHO), Cuba reported 873 cases of Zika. The PAHO doesn’t have data for Cuba from 2019 as of the date of publication of this article. In addition, Puerto Rico reported 39 cases .
In other parts of the Caribbean, the disease has seen a decline. For example, the U.S. Virgin Islands reported their last confirmed cases in June 2018, Saint Lucia reported its last cases in 2016, and Grenada, Anguilla and Dominican Republic reported their last cases in 2017.
Below are the countries and territories in the Caribbean that have a current risk of Zika:
South America
In 2019, according to the PAHO , Brazil reported 3,323 cases of Zika. After Brazil, the countries in South America that reported the majority of cases were Peru (829) and Bolivia (27).
Below are the countries in South America that have a current risk of Zika:
Pacific Islands
In 2018, Australian health authorities reported 2 cases where the probable places of infection were Fiji and Vanuatu. Overall, while large outbreaks occurred in this area in 2014, new cases have been rare.
Below are the Pacific islands that have a current risk of Zika:
Currently, India is the area with the most cases being currently reported in Asia . As of November 2018, 159 confirmed cases were reported in 2018.
Thailand has also reported cases in recent years. In 2018, 568 cases were reported across the country and in 2019, 48 cases have been reported as of June.
Media outlets have also reported cases with probable infection in Vietnam, Malaysia, the Philippines, Singapore, Indonesia, and the Maldives.
Below are the countries in Asia that have a current risk of Zika:
Multiple countries in Africa are considered to have current or past Zika transmission. In recent years, Zika has been reported in Angola, Cabo Verde, and Guinea Bissau.
According to the WHO Eastern Mediterranean Regional Office, no cases have been recorded in the countries since August 2019. However, since not all areas report health information, it is difficult to truly gauge the current risks in Africa.
Below are the countries in Africa that have a current risk of Zika:
Bottom Line: Real-time data on Zika Virus outbreaks and transmission is often not available. This is because most people who become infected with Zika do not show signs or symptoms. In some countries, reliable reporting and monitoring systems that track virus transmission may not be available.
There is currently no vaccine to prevent Zika. There is research in progress that may lead to a vaccine in the future, though.
The National Institute of Allergy and Infectious Disease (NIAID) is currently in phase 2 of a Zika vaccine trial in Texas, Puerto Rico, and South and Central America [ 8 ]. Other vaccine trials exist, too, but may take years to finalize [ 9 ].
Beyond simply avoiding those countries, here are some strategies from the CDC to avoid Zika if you do travel to a country that has or is at risk of having Zika.
Mosquito Control
The single best way to prevent diseases spread by mosquitoes is to protect yourself and your family from mosquito bites.

According to the CDC, here are a few preventative measures to take:
- When you go into mosquito-infested areas, wear a long-sleeved shirt and long pants
- Treat your clothing and gear with permethrin or buy pre-treated items
- Wear socks and shoes instead of sandals
- Use Insect Repellent
- DEET (note that a higher concentration of DEET doesn’t mean a product is stronger, only that it lasts longer)
- Oil of lemon eucalyptus (OLE)*
- Para-menthane-diol (PMD)*
- 2-undecanone
*The CDC recommends not using these products on children under 3 years old.
Apply insect repellent based on the instructions used on the container’s label. EPA-registered insect repellents are proven safe and effective for pregnant and breastfeeding women but are not approved for use on babies younger than 2 months old.
The CDC does not recommend natural insect repellents (such as those made with citronella, lemongrass, peppermint, and cedarwood).
Hot Tip: Parents should apply insect repellent to their children. It should be applied only to exposed skin. Avoid hands, eyes, cuts, or irritations. You might want to put it on your hands first, then rub it on your child so you don’t use too much.
Make Yourself a Less-Attractive Target
Don’t use scented lotions or sprays that can attract bugs before going outside.
You may also have heard people say that they always get bit while others are left without a trace. There may be some truth to this! The main way mosquitoes search for their next victim is by carbon dioxide output.
Unfortunately, most of this directly relates to genetics but you do have some control. Drinking alcohol and exercising can both raise your resting metabolic rate and carbon dioxide output, making you more attractive to mosquitoes [ 10 ].
Stay in Air-Conditioned or Well-Screened Housing
The mosquitoes that carry the Zika virus are most active from dawn until dusk, but they can also bite at night. Stay in places with air conditioning and keep windows and doors closed.
If this is not possible, ensure that the doors and windows have screens to keep mosquitoes out.
Take Steps to Control Mosquitoes Outside Your Home
The goal is to reduce the breeding habitat to lower the overall mosquito population. The mosquitoes that carry the Zika virus typically breed in standing water that can collect in such things as animal dishes, flower pots, and used car tires.
Make sure to dump out water from smaller containers periodically, but especially after rainfall. Cover larger bodies of water like pools and hot tubs when not in use.
Sleep Under a Mosquito Bed Net
If air-conditioned or screened rooms are not available or if you are sleeping outdoors, but sure to sleep under a mosquito net. Mosquito netting can also be used to cover babies younger than 2 months old in their strollers or cribs as using an insect repellent is not safe for them.
Here are some guidelines for using a mosquito net:
- Tuck the net under the mattress to keep the mosquitoes out
- Tuck netting over a crib under the mattress or select a net long enough to touch the floor
- Pull the net tight to avoid choking hazards for young children
- Check for holes or tears in the net where mosquitoes can enter
- Do not sleep directly against the net, as mosquitoes can still bite through holes in the net
Hot Tip: Do not hang the mosquito net near open flames or candles as it can catch on fire.
Protected Sex
Prevent sexual transmission of Zika by using condoms or not having sex for either the rest of the pregnancy or for 3 months after return from a country with current or past known transmissions.
This advice applies to both men and women. Men can transmit Zika to their partner, who can then, in turn, transmit to a baby.
Blood Transfusion
According to the CDC, there have been no confirmed transfusion-related transmission cases of Zika virus in the U.S. [ 11 ]. However, cases of Zika virus transmission through platelet transfusions have been documented in Brazil.
There isn’t anything in particular individuals can do to prevent transmission through blood transfusions. All blood donations are now screened for the Zika virus and the CDC notes that they ensure “the blood supply safe is by assisting state and local health departments and hospitals in investigating reports of potential infectious disease transmission.”
Bottom Line: The only way to protect yourself from the Zika virus is to take preventative measures regarding mosquitoes and having protected sex.
As many as 4 out of 5 people infected with the Zika virus have no signs or symptoms. When symptoms do occur, they usually begin 2 to 7 days after a person is bitten by an infected mosquito.
According to the Mayo Clinic, the symptoms include [12]:
- Joint or muscle pain
- Red eyes (conjunctivitis)
People typically recover in about a week and death is rare.
If you have traveled to an area with known Zika outbreaks and develop some of the common symptoms noted above, please go see your healthcare provider immediately. A blood or urine test can identify a Zika infection .
Be sure to tell them where you traveled and whether or not you may be pregnant. In addition, if you are taking medicine for another medical condition, talk to your doctor before taking additional medication.
Bottom Line: We are not medical professionals, so always discuss all of these items with your healthcare provider.
For most of those infected, with the exception of babies, the side effects of Zika are not usually severe. Common practice has been to treat symptoms as there is no “cure” for Zika.
After you see your doctor, they may tell to do some of the following items in order to treat the symptoms:
- Get plenty of rest
- Drink fluids to prevent dehydration
- Take medicine such as acetaminophen to reduce fever and pain
- Do not take aspirin or other non-steroidal anti-inflammatory drugs (NSAIDs)
How Long is Zika Detectable in the Body?
According to a study [ 13 ], Zika was still detectable in 5% or more of participants up to:
- Vaginal fluids — 7 days
- Saliva — 7 days
- Urine — 39 days
- Blood — 54 days
- Semen — 3 months
The good news is that once a person has been infected with Zika, they are likely to be protected from future infections.
Guillain-Barre Syndrome
As briefly touched on earlier, there is also a 20 fold increased risk of Guillain-Barre syndrome. This is an autoimmune disease that results in widespread weakness and paralysis. While it only occurs in less than 1% of Zika infections, it can have severe complications.
Two-thirds of patients lose the ability to walk, and 25% need to be put on a mechanical ventilator because of the weakness of the respiratory muscles. Those with the syndrome usually experience their most significant weakness within 2 to 4 weeks after symptoms begin.
According to the Mayo Clinic [ 14 ], signs and symptoms of Guillain-Barre syndrome may include:
- Prickling, pins and needles sensations in your fingers, toes, ankles or wrists
- Weakness in your legs that spreads to your upper body
- Unsteady walking or inability to walk or climb stairs
- Difficulty with eye or facial movements, including speaking, chewing or swallowing
- Severe pain that may feel achy or cramp-like and may be worse at night
- Difficulty with bladder control or bowel function
- Rapid heart rate
- Low or high blood pressure
- Difficulty breathing
There’s no known cure for Guillain-Barre syndrome, but several treatments can ease symptoms and reduce the duration of the illness. Most people make a partial or full recovery.
Studies have found that pregnant women have 2 disadvantages when it comes to mosquitoes [ 15 ]. Their body temperatures tend to be higher and they also exhale more carbon dioxide, both of which can lead to more mosquito bites.
If a pregnant woman gets bitten and contracts the Zika virus, she may transmit it to her baby. Babies are much more likely to be born with a birth defect in which a developing baby’s brain fails to grow to its usual size. Hearing and vision problems have also been reported in newborns exposed to Zika in the womb.
Because of this, the CDC recommends special precautions for pregnant women. In addition, if you are considering getting pregnant, partners will need to take precautions as well.

Trying to Conceive
Women who have had symptoms of the virus or tested positive for it should wait at least 8 weeks after their symptoms first appeared before trying to get pregnant according to the CDC.
In addition, the CDC recommends that men who had symptoms should wait between 3 to 6 months before having unprotected sex. The reason for the difference in time is because Zika can be found in semen longer than in other body fluids.
Already Pregnant
If your partner has Zika or has traveled in an area with an outbreak, continue to follow the same guidelines as above – no unprotected sex for 3-6 months. This will prevent transmission while the mother is carrying the child.
If Zika is transmitted, the infection during pregnancy can cause a birth defect of the brain called microcephaly. It is also linked to other problems, such as miscarriage, stillbirth, and other birth defects.
Estimates of the rate of birth defects related to Zika have ranged from 6% to 42%. Additionally, while studies are being done to determine more information about the effects of contracting the virus at different stages in pregnancy, what is known is that the earlier the infection occurred in pregnancy, the greater the risk.
If families would like to speak to someone about a possible Zika virus infection or diagnosis during pregnancy and risk to the baby, the CDC has a free and confidential service available called MotherToBaby. This service available Monday-Friday 8 a.m. – 5 p.m. (local time) at:
- Call 866-626-6847 or text 855-999-3525
- Chat live or send an email through the MotherToBaby website
Future Pregnancies
Based on the available evidence, the CDC believes that the Zika virus infection in a woman who is not pregnant would not pose a risk for birth defects in future pregnancies after the virus has cleared from her blood.
From what is known about similar infections (like West Nile and Yellow Fever), once a person has been infected with Zika virus, he or she is likely to be protected from a future Zika infection.
If you have been infected with Zika previously, it is always best to consult your doctor before trying to conceive again.
Babies who were infected with Zika before birth may have damage to their eyes and the part of their brain that is responsible for vision, affecting their visual development. Both babies with and without microcephaly can have eye problems.
If your baby was born with a congenital Zika infection, he or she should receive the CDC-recommended screenings and tests to check for eye and other health problems, even if your baby appears healthy.
Much is still unknown about how Zika affects those babies born with or without microcephaly. Even without the obvious birth defect or for mothers that never showed symptoms, there may still be other side effects.
Studies are being done which show that there might be detrimental effects on long-term memory, social interactions, and abstract reasoning as patients age [ 16 ].
Many couples choose to take babymoons to relax and enjoy time together before the arrival of their new bundle of joy. While it may sound appealing to head to a tropical destination for some sun and sand, it is also important for expectant mothers to avoid countries with a high risk of Zika.
There are still plenty of vacation-worthy destinations to visit if you’re looking to travel somewhere without any reported Zika cases! Some great options include Bermuda, Morocco, Canary Islands, Mauritius, New Zealand , Chile, Azores, Hawaii , Seychelles , and most of Europe .
Other Factors to Consider
If you’re looking to go somewhere else, be sure to think about the specific areas and times you want to travel to better assess your risk of contracting Zika.
Higher-altitude areas tend to have fewer mosquitoes. In addition, look into what season you would be traveling during. Rainy season is often when the transmission of mosquito-borne viruses is at its highest.
Whenever you arrive at your destination, try to get rooms on higher floors and check the screens on the windows. If there aren’t any, be sure to keep them closed to avoid bringing the mosquitoes in the room.
Hot Tip: Travel insurance can go a long way toward providing peace of mind during any trip. If you get ill while traveling, you’ll find consolation in being financially covered. With our complete guide to travel insurance , you’ll find the best affordable travel coverage and get tips for finding travel coverage you might already have.
Overall, Zika is on the decline, but this doesn’t mean you can forget about the potential risks. It is always good to be informed and take the preventative measures we’ve laid out above.
Pregnant women and couples planning on having kids in the near future should be especially aware of the risks of Zika when choosing travel destinations. It may be good to postpone or change travel plans to prevent any serious complications.
All information and content provided by Upgraded Points is intended as general information and for educational purposes only, and should not be interpreted as medical advice or legal advice. For more information, see our Medical & Legal Disclaimers .
UP's Bonus Valuation
This bonus value is an estimated valuation calculated by UP after analyzing redemption options, transfer partners, award availability and how much UP would pay to buy these points.
- Skip to main content
- Skip to "About this site"
Language selection
Search travel.gc.ca.
Help us to improve our website. Take our survey !
Zika virus: Advice for travellers
Level 2 - practise enhanced health precautions ( more details ).
Original publication date: January 28, 2014
Updated: August 31, 2023
- The Current Situation and Health Professionals sections have been updated.
Current Situation
Zika virus continues to be a concern in many parts of the world. Transmission can occur in most areas of the world where the mosquito Aedes aegypti, the principal vector, occurs. This means that there is the potential for transmission through much of the tropical and subtropical world and beyond.
The risk of transmission to travellers is considered low.
To find out if your destination is a country or area with risk of Zika virus, consult the Travel Advice and Advisories page , and select your destination. Information on diseases spread by insects, such as Zika virus, is found under the ‘Health’ tab.
Zika virus typically causes mild illness lasting only a few days. Many people who are infected have no symptoms and do not know that they have been infected. Only 1 in 4 people infected with Zika virus develop symptoms.
Symptoms of Zika virus infection often include:
- conjunctivitis (pink eye)
- joint and muscle pain
A Zika virus infection in a pregnant woman can pose significant risks to the unborn baby, even if the woman does not develop any symptoms. Zika virus can cause serious birth defects including microcephaly (an abnormally small head), brain abnormalities, vision and hearing loss, and more. When some of these birth defects are present together, the condition is called Congenital Zika Syndrome (CZS).
There have also been increased reports of a serious nervous system disorder in adults, called Guillain-Barré syndrome , in countries and areas with risk of Zika virus.
Zika virus is primarily spread through the bite of an infected mosquito. It can also spread by:
- A pregnant woman infected with Zika virus passing the virus to her unborn baby.
- A person infected with Zika virus passing the virus through sexual contact. This includes contact with semen, vaginal fluid, blood or other body fluids during vaginal, anal or oral sex without a condom. This may also include the sharing of sex toys.
- A person infected with Zika virus who donates cells, blood, tissue, sperm (semen) or organs.
Currently, there is no vaccine to prevent or medication to treat infection with Zika virus. Symptoms, when present, will typically resolve on their own within a few days. Treatment aims to relieve symptoms.
Recommendations
For pregnant women or those planning a pregnancy
Zika virus infection during pregnancy increases the risk for serious birth defects since women can pass the virus to their unborn babies.
Pregnant women and women planning a pregnancy should visit a health care professional at least 6 weeks before travelling to discuss the potential risks of travelling to a country or area with risk of Zika virus. Pregnant women may choose to avoid or postpone travel to these areas.
Zika virus can be sexually transmitted. Infected men with or without symptoms, can carry Zika virus in their semen for a prolonged period of time. Partners should be aware of the risk so they can make informed travel decisions and take appropriate precautions.
Pregnant women should always use condoms correctly or avoid sexual contact with anyone who has travelled to a country or area with risk of Zika virus for the duration of their pregnancy.
For all travellers to countries or areas with risk of Zika virus
Before your trip
- Consult a health care professional or visit a travel health clinic at least 6 weeks before you travel.
During your trip
- Use approved insect repellent and apply it properly
- Cover up by wearing light-coloured, loose clothing, long pants and tucked-in long-sleeved shirts with closed-toe shoes or boots and a hat.
- Sleep in indoor areas that are completely enclosed or well-screened.
- Use mosquito netting (bed net) when sleeping outdoors or staying in a building that is not completely enclosed and to cover playpens, cribs or strollers.
- Learn more about mosquito bite prevention for travellers .
- Always use condoms correctly or avoid sexual contact while in countries or areas with risk of Zika virus.
After your trip
- See a health care professional if you had or currently have symptoms of Zika virus infection .
- Tell your health care professional:
- where you have been living or travelling, and
- if you have had unprotected sexual contact with someone who could be infected with Zika virus.
- Always use condoms correctly or avoid sexual contact for 2 months after travel or after onset of illness due to Zika virus (whichever is longer).
- Before trying for a pregnancy, wait 2 months after travel or after onset of illness due to Zika virus (whichever is longer) , to reduce the risk of passing the virus to your unborn baby. If your male partner travelled with you, wait 3 months after travel or after onset of illness due to Zika virus (whichever is longer) , to reduce the risk of sexual transmission.
- If you have a pregnant partner, always use condoms correctly or avoid sexual contact for the duration of the pregnancy.
- Before trying for a pregnancy or donating semen, wait 3 months after travel or after onset of illness due to Zika virus (whichever is longer) , to reduce the risk of sexual transmission.
- In all other situations, always use condoms correctly or avoid sexual contact for 3 months after travel or after onset of illness due to Zika virus (whichever is longer).
Information for Health Care Professionals
- The Committee to Advise on Tropical Medicine and Travel (CATMAT) has developed a statement on Prevention and Treatment of Zika virus.
- Zika virus: For health professionals
Registration of Canadians Abroad
Sign up with the Registration of Canadians Abroad service to stay connected with the Government of Canada in case of an emergency abroad or an emergency at home.
- World Health Organization - Zika virus classification tables
- World Health Organization - Zika virus disease
- Government of Canada – Zika virus
- Safer condom use
- If you become sick or injured while travelling outside Canada or after your return

European Centre for Disease Prevention and Control
An agency of the European Union
- Infectious disease topics
- Zika virus infection
- Surveillance and disease data
- Travel-associated Zika virus disease cases
Travel-associated Zika virus disease cases: place of infection of cases imported to the EU/EEA
The maps and table below show the places where travel-associated Zika virus disease cases reported to ECDC were likely to have been infected. The aim is to inform public health authorities in the European Union/European Economic Area (EU/EEA) and their citizens of the risk related to Zika virus disease cases.
Distribution of travel-associated Zika virus disease cases reported to ECDC, by place of infection, 2022

Distribution of travel-associated Zika virus disease cases reported to ECDC, by place of infection, 2018–2022

About the data
The report is exclusively based on the places of infection linked to confirmed Zika virus disease cases, reported to ECDC by EU/EEA countries through The European Surveillance System (TESSy), as of June 2024. Only cases diagnosed in the EU/EEA were reported.
Data on place of infection were provided at the sub-national level for the EU outermost regions*, while for other places data are at national level. Cases infected in the EU outermost regions were classified as travel-associated and were not included in the national count for France, Portugal or Spain. Locally acquired and travel-associated cases infected within mainland EU/EEA were excluded.
Information on the transmission events occurring within mainland EU/EEA are described in ECDC’s Annual Epidemiological Reports .
In this summary, in order to remove outliers, we included the places of infection linked to confirmed cases which were identified over the past five years (i) in at least two different years and (ii) in at least two different EU/EEA countries.
The data presented in this summary should be interpreted with caution for the following reasons:
- the data were included as reported by EU/EEA countries and were not validated by the health authorities at the places of infection;
- this summary does not account for regional disparity within the places of infection, despite some regions having a higher risk of infection than others;
- the data presented only cover the years 2018−2022 and therefore do not imply ongoing transmission. Data from 2023 are currently being validated and will be made available by the end of 2024.
About Zika virus disease
Zika virus disease is transmitted by Aedes mosquitoes to humans. Zika virus disease is not endemic in mainland Europe and the vast majority of cases are travellers infected outside of mainland Europe. Zika virus disease is a notifiable disease at EU level and surveillance data is collected by ECDC through TESSy.
*EU outermost regions include Guadeloupe, French Guiana, Réunion, Martinique, Mayotte and Saint-Martin (France), the Azores and Madeira (Portugal), and the Canary Islands (Spain).
Zika-Free Caribbean Islands

The worst thing that can happen while traveling abroad is getting sick. Every year, it seems a new virus is emerging, and since 2016, we are ‘blessed’ with the Zika Virus. This nasty virus is spreading to remote and gorgeous Caribbean islands.
The Centers for Disease Control and Prevention (CDC) keeps an up to date list of All Countries and Territories with Active Zika Virus Transmission , that are still dangerous to visit, especially for pregnant women.
As stated by the CDC, “because Zika infection during pregnancy can cause severe birth defects, pregnant women should not travel to the areas below. Partners of pregnant women and couples considering pregnancy should know the risks to pregnancy and take prevention steps. All travelers should strictly follow steps to prevent mosquito bites and prevent sexual transmission during and after the trip.”
On one hand, on August 14, 2018, the Caribbean Public Health Agency (CARPHA) released a statement concluding “ that Zika virus transmission in the Caribbean Region has been interrupted, and that the risk to residents and visitors to the Region of acquiring Zika is low “.
On the other hand, the CDC updated its travel recommendations on September 5, 2018, listing the Caribbean Islands where there is still a risk of getting infected by the Zika virus. Only the islands listed below are Zika free according to the CDC.
So you can choose which source of information you want to trust: the CARPHA or the CDC. The decision is up to you and the consequences of getting infected are very different if you are pregnant, want to become pregnant or not. I am only giving you the information so you can make a clear decision.
Looking at a map, it looks like there are no countries risk-free in the Caribbean. But rest assured: there’s still some safe paradise islands in the Caribbean Sea and Region.
Discover the last Zika free Caribbean islands and destinations, and don’t forget to download the infographic at the end of this article!
Your Custom Travel World Map

Travel, and maps , are the only things you buy that make you richer, don’t you agree?
Customize your own modern and minimalist hand-drawn large size travel world map, and get free wooden push pins and all flags of the world complimentary! Free shipping in USA and Canada.
-> Click here to customize yours now <-
Zika free caribbean islands and destinations, bermuda: zika free islands.

I know, Bermuda isn’t located in the Caribbean Sea. But it isn’t that far, so I’ve decided to include it in this list of safe destinations since it is. Truly isolated, Bermuda is famous for its pink sand dazzling beaches, tidy pastel cottages and the namesake ‘triangle’ sucking in hapless ships and planes. Don’t worry, you will come back from Bermuda alive and well!
Caribbean Destinations with Current or Past Transmission But No Zika Outbreak
Unfortunately, the destinations below are or have been infected with the Zika Virus. Please take note that there is no CURRENT outbreak: so before visiting, talk to a health care provider about potential risks. If you decide to travel, prevent mosquito bites and sexual exposure to Zika.
Antigua & Barbuda
Updated on august 23, 2016.

Antigua is a bustling island that gets visited by celebrities, such as Eric Clapton, Oprah, and more. Some people say Antigua has 365 beaches, one for every day of the year. Hard to verify, but since there are so many powdery white sand beaches, we can surely find one to be our own private paradise for a day!
Antigua & Barbuda Travel Guide
Updated on April 6, 2019

With more than 3000 islands and limestone rocks in the glistening turquoise Caribbean Sea, the Bahamas offer a wide variety of landscapes and adventures for everyone: from the vibrant Nassau to the forgotten Out Islands. Here you can find casinos, luxury yachts, golf courses and rum.
The Bahamas Travel Guide
Updated on April 23, 2016

Belize is a tiny country, boarding the Caribbean Sea, but it has so much to offer. From its famous Barrier Reef and Blue Hole to finger-licking ceviche and very friendly English-speaking inhabitants, a trip to Belize is without a doubt unforgettable (I know it, I’ve been there!).
Belize Travel Guide
Cayman Islands: The Travelers Must Be Cautious
The Cayman Islands are officially on the CDC’s Zika-Free list. Nonetheless, rumors have been spreading that there might be a very small risk of the Zika virus on the Cayman Islands. So travelers must be cautious: even though the CDC declared the Cayman Islands Zika free, there might be an undeclared risk of a Zika virus presence.
Well-known for its incredible scuba diving and snorkeling sites, Grand Cayman is the largest of the Cayman Islands, a British Overseas Territory. But don’t forget beautiful Cayman Brac, which is popular for their stunning deep-sea fishing excursions, and also Little Cayman (the smallest of the 3 islands), that’s home to diverse wildlife, from endangered iguanas to seabirds such as red-footed boobies.
Updated on June 7, 2016

Grenada seems underrated, compared to other Caribbean splendid islands. But don’t be fooled: you won’t be disappointed with a trip here! Its beaches are made of pure white glowing sand, the diving is delightful, and the landscapes are visions of lush, tropical, idyllic hillsides.

Guadeloupe is an archipelago of over a dozen islands that offer gorgeous beaches to amazing mountains. The two main islands look like the wings of a butterfly and are joined together by a couple of bridges and a mangrove swamp.
Grande-Terre, the eastern of the two islands, has a string of beach towns that offer travelers plenty of activities to do and breathtaking beaches. Basse-Terre, the western island, is more mountainous and it is home to the beautiful Parc National de la Guadeloupe, which is crowned by the spectacular La Soufrière volcano.

Martinique is an overseas region of France and its culture is a blend of French and West Indian culture. That’s why you’ll find here top-notch culinary experiences and rich cultural life, while its volcanic origin offers a striking diversity of landscapes, superb beaches, and stunning hiking.
St. Barts (Saint Barthélemy)

Saint Barthelemy, a French-speaking Caribbean island well-known as St. Barts, offers travelers white-sand beaches and trendy designer shops. Gustavia, its capital, is postcard-perfect with a yacht-filled harbor, delicious high-end restaurants, and educational historical attractions.
Saint Kitts & Nevis
Updated on october 15, 2016.

Looking for amazing snorkeling, a laid-back attitude and fine beaches? Look no more! Saint Kitts still maintains its easy-going-ness, even if it’s used to tourist crowds now.
Saint Lucia

Rich, lush tropical hillsides, pure white sand beaches and dazzling blue sea are what you will find in Saint Lucia. The Pitons are obviously a landscape that will decorate your desktop computer for a while!
Turks & Caicos
Updated on august 23, 2016.

Last, but not the least, Turks and Caicos are a group of 40 islands dotting their own patch of warm water just north of the Caribbean Sea and south of the Bahamas. Even if these islands are located close to Miami, the crowds haven’t taken over the pristine landscapes and friendly inhabitants.
List of all the countries where the Zika virus is active in the Caribbean region
Here’s an exhaustive list of all the countries in the world with current or past transmission but no zika outbreak.
American Samoa, Angola, Anguilla, Antigua and Barbuda, Argentina, Aruba, Bahamas, Bangladesh, Barbados, Belize, Bolivia, Bonaire, Brazil, British Virgin Islands, Burkina Faso, Burma, Burundi, Cambodia, Cameroon, Cape Verde, Cayman Islands, Central African Republic, Colombia, Cook Islands, Costa Rica, Cuba, Curacao, Dominica, Dominican Republic, Easter Island, Ecuador, El Salvador, Ethiopia, Federated States of Micronesia, Fiji, France, French Guiana, French Polynesia, Gabon, Grenada, Guadeloupe, Guatemala, Guinea-Bissau, Guyana, Haiti, Honduras, India, Indonesia, Ivory Coast, Jamaica, Laos, Malaysia, Maldives, Marshall Islands, Martinique, Mexico, Montserrat, New Caledonia, Nicaragua, Nigeria, Palau, Panama, Papua New Guinea, Paraguay, Peru, Philippines, Puerto Rico, Saba, Saint Barthelemy, Saint Kitts and Nevis, Saint Lucia, Saint Martin, Saint Vincent and the Grenadines, Samoa, Senegal, Singapore, Sint Eustatius, Sint Maarten, Solomon Islands, Suriname, Thailand, Tonga, Trinidad and Tobago, Turks and Caicos, Uganda, United States (Continental US), United States Virgin Islands, Vanuatu, Venezuela, Vietnam
Here’s a list of the countries in the world with mosquitoes but no reported Zika cases
Australia, Benin, Bhutan, Botswana, Brunei, Chad, China, Christmas Island, Congo, Democratic Republic of Congo, Djibouti, East Timor, Egypt, Equatorial Guinea, Eritrea, Georgia, Ghana, Guam, Guinea, Kenya, Kiribati, Liberia, Madagascar, Madeira Islands, Malawi, Mali, Mozambique, Namibia, Nauru, Nepal, Niger, Niue, Northern Mariana Islands, Oman, Pakistan, Russia, Rwanda, Saudi Arabia, Sierra Leone, Somalia, South Africa, South Sudan, Sri Lanka, Sudan, Taiwan, Tanzania, The Gambia, Togo, Tokelau, Turkey, Tuvalu, Uruguay, Wallis and Futuna, Yemen, Zambia, Zimbabwe
Here’s a list of all the countries in the world with no mosquitoes that spread Zika
Afghanistan, Albania, Algeria, Andorra, Armenia, Austria, Azerbaijan, Azores, Bahrain, Belarus, Belgium, Bermuda, Bosnia and Herzegovina, British Indian Ocean Territory, Bulgaria, Canada, Canary Islands, Chile, Cocos Islands, Comoros, Corsica, Croatia, Crozet Islands, Cyprus, Czech Republic, Denmark, Estonia, Eswatini, Falkland Islands, Faroe Islands, Finland, Germany, Gibraltar, Greece, Greenland, Guernsey, Hong Kong, Hungary, Iceland, Iran, Iraq, Ireland, Isle of Man, Israel, Italy, Japan, Jersey, Jordan, Kazakhstan, Kerguelen Islands, Kosovo, Kuwait, Kyrgyzstan, Latvia, Lebanon, Lesotho, Libya, Liechtenstein, Lithuania, Luxembourg, Macau, Malta, Mauritania, Mauritius, Mayotte, Moldova, Monaco, Mongolia, Montenegro, Morocco, Netherlands, New Zealand, Norfolk Island, North Korea, North Macedonia, Norway, Pitcairn Islands, Poland, Portugal, Qatar, Reunion, Romania, Saint Helena, Saint Paul and New Amsterdam Islands, Saint Pierre and Miquelon, San Marino, São Tomé and Principe, Serbia, Seychelles, Slovakia, Slovenia, South Georgia and the South Sandwich Islands, South Korea, Spain, Sweden, Switzerland, Syria, Tajikistan, Tunisia, Turkmenistan, Ukraine, United Arab Emirates, United Kingdom, Uzbekistan, Vatican City, Wake Island, Western Sahara
Zika Free Caribbean Islands: Will it get better one day?
According to experts, since the Zika virus is found in tropical regions, there are low chances that it will stop spreading. The best way to stay safe is to protect yourself from being infected with the virus .
Have you or one of your family member been infected by the Zika virus?
Personalize your Own Push Pin World Map Now!

Travel, and maps , are the only things you buy that make you richer.
Modern and minimalist hand-drawn large size travel world map: it includes the names of the countries, Canadian provinces, US States, seas and oceans in ENGLISH.
Click here to customize yours now!

Unfortunately, it looks like The Bahamas are now on the active Zika virus transmission list as well 🙁
Thank you SO MUCH Shannon for the update! I just looked it up, and you are right! Thanks 😉
Thank you for crossing out when up-dates come in
You are very welcome! That’s the least I can do!! 😉
This content is really interesting. I have bookmarked it. Do you allow guest post on your website ? I can provide hi quality articles for you. Let me know.
Yes, I do welcome guest posts! Here’s my Guest Post Policy: https://www.easyplanettravel.com/easy-planet-travel-guest-post-policy/ Hope to read you soon!
Wow! After seeing your article, i actually want to go to there immediately. It is very nice. I like…
Thanks a lot!
What an amazing place 😀 thanks for sharing!! We will definitely add it to our travel list.
Happy to help 🙂
Yeah, this is an amazing point of destination! It is a must visit place! Undoubtedly! Thank you very much for introducing this issue!
You are welcome 😉
Leave a Comment Cancel reply
- Free Minimalist World Map
- Free Bilingual World Map
- Travel Guides
- Travel Costs
- Travel Adapters
- Where to Go When
- Destinations By Interest
- Media Contact
- Get in touch

- Canadian dollar ($) - CAD
- United States (US) dollar ($) - USD

C O N G R A T U L A T I O N S,
you've unlocked
your 1st order!
You'll receive your 10% off
coupon code by email within
the next 15 minutes.
Happy shopping!
We’re sorry, this site is currently experiencing technical difficulties. Please try again in a few moments. Exception: request blocked
- Skip to main content
- Skip to FDA Search
- Skip to in this section menu
- Skip to footer links

The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you're on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
U.S. Food and Drug Administration
- Search
- Menu
- Emergency Preparedness and Response
- Counterterrorism and Emerging Threats
- Medical Countermeasures Initiative (MCMi)
Zika Virus Response Updates from FDA

Fast Facts : About Zika | Locations Affected | Guillain-Barré Syndrome | Pregnancy | Medical Products | Prevention
Zika Information from FDA : Updates by Date | Safety of the Blood Supply | Emergency Use Authorization | Investigational Products | Fraudulent Products | Using Insect Repellants Safely | Events | More About FDA’s Role | Contact FDA | Related Links | Resources for Healthcare Providers | Translations (Spanish, Portuguese)
Zika virus is spread to people primarily through the bite of an infected Aedes species mosquito. Most people never know that they have been infected with the virus. It is estimated that four out of five people with Zika virus infections have no symptoms at all. When symptoms do occur, the most common symptoms are fever, rash, joint pain, and conjunctivitis (red eyes). Even in those who develop symptoms, the illness is usually mild, with symptoms lasting from several days to a week.

A pregnant woman applies mosquito repellant. Using insect repellants will help to protect her from being bitten by a mosquito that may be carrying a virus such as Zika; this will also protect her unborn baby from the virus. (Image: CDC/Division of Vector-borne Diseases)
Locations Affected
Prior to 2015, Zika virus outbreaks had occurred in areas of Africa, Southeast Asia, and the Pacific Islands. However, in May 2015, the Pan American Health Organization (PAHO) issued an alert (PDF, 199 KB) regarding the first confirmed Zika virus infection in Brazil. For information on current outbreaks, see from CDC:
- Zika in the US
- Zika Travel Information
Guillain-Barré Syndrome
Since the outbreak in Brazil began, we have seen reports of Guillain-Barré syndrome (a disorder in which the immune system attacks the nervous system) and birth defects. More: Zika and Guillain-Barré Syndrome , from CDC
Zika virus can be transmitted from a pregnant mother to her fetus. Scientists at the Centers for Disease Control and Prevention (CDC) concluded , after careful review of existing evidence, that Zika virus is a cause of microcephaly , a condition in which a baby’s brain and head is smaller than expected, and other severe fetal brain defects. In the April 13, 2016 report published in the New England Journal of Medicine , the CDC authors describe a rigorous weighing of evidence using established scientific criteria.
The finding that Zika virus infection can cause microcephaly and other severe fetal brain defects means that a woman who is infected with Zika during pregnancy has an increased risk of having a baby with these health problems. It does not mean, however, that all women who have Zika virus infection during pregnancy will have babies with problems. As has been seen during the current Zika outbreak, some infected women have delivered babies that appear to be healthy. More: Zika and pregnancy, from CDC , and CDC updates guidance for infants born to mothers with possible Zika virus infection during pregnancy (October 19, 2017)
Preventing pregnancy: If you decide that now is not the right time to have a baby, talk to your healthcare provider. View information on the safety and effectiveness of FDA-approved medicines and devices for birth control (en Español Guía de Métodos Anticonceptivos (PDF, 433 KB))

Medical Products
There are no FDA-approved vaccines for Zika virus. Several investigational vaccines are under development, including early human clinical trials .
There are no FDA-approved treatments for Zika virus , nor is the FDA aware of treatments in advanced development for Zika at this time. Also see Zika Virus Treatment Research from NIAID
Diagnostics: FDA-authorized diagnostic tests for detecting Zika virus antibodies:
- ZIKV Detect 2.0 IgM Capture ELISA - On May 23, 2019, FDA authorized marketing of the ZIKV Detect 2.0 IgM Capture ELISA to detect Zika virus immunoglobulin (IgM) antibodies in human blood. The ZIKV Detect 2.0 IgM Capture ELISA is the first Zika diagnostic test the FDA has allowed to be marketed in the U.S. FDA reviewed the data for the test through the De Novo premarket review pathway. Previously, tests for detecting Zika virus immunoglobulin (IgM) antibodies—including the ZIKV Detect 2.0 IgM Capture ELISA—had been authorized only for emergency use under the FDA’s Emergency Use Authorization (EUA) authority. For more information, see Serological assays below
- ADVIA Centaur Zika test – On July 17, 2019, FDA cleared the ADVIA Centaur Zika test. This is the second Zika diagnostic test FDA has allowed to be marketed in the U.S. for detecting Zika virus IgM antibodies. Previously, the test had been authorized only for emergency use under FDA’s EUA authority.
- LIAISON XL Zika Capture IgM Assay II – On October 28, 2019, FDA cleared the LIAISON XL Zika Capture IgM Assay II for detecting Zika virus IgM antibodies. Previously, the test had been authorized only for emergency use under FDA’s EUA authority.
- DPP Zika IgM Assay System – On June 3, 2020, FDA cleared a similar DPP Zika IgM System for detecting Zika virus IgM antibodies. Previously, the test had been authorized only for emergency use under FDA’s EUA authority.
FDA encourages commercial diagnostic developers and researchers developing laboratory developed tests for Zika virus to submit an EUA request or consider pursuing a premarket submission. FDA will work interactively with developers to support such requests. See Zika Virus Emergency Use Authorization for information about Zika virus diagnostics available under EUA.
FDA stands ready to work with medical product developers to clarify regulatory and data requirements necessary to move products forward in development as quickly as possible.
See also: Zika Symptoms, Diagnosis, & Treatment, from CDC
The best way to prevent Zika and other diseases spread by mosquitoes is to avoid being bitten. More: Prevention, from CDC
Zika Information from FDA
Updates by date, latest updates.
- May 20, 2024: Information for Human Cell, Tissue, and Cellular and Tissue-Based Product (HCT/P) Establishments Regarding FDA’s Determination that Zika Virus is no Longer a Relevant Communicable Disease Agent or Disease (RCDAD) - Because Zika virus (ZIKV) is no longer an RCDAD, HCT/P establishments may discontinue screening donors for ZIKV and revise their relevant procedures to reflect this change.
- March 9, 2023: FDA held a Grand Rounds lecture: Microphysiological Systems as Novel Disease Models and Drug Development Tools . This presentation by researchers from FDA's National Center for Toxicological Research (NCTR) evaluates nonhuman primate testicular organoids for use as an in vitro model of Zika virus infection. A recording is available.
Additional updates (2021 and earlier)
May 12, 2021: Information for Blood Establishments Regarding FDA’s Determination that Zika Virus is no Longer a Relevant Transfusion-Transmitted Infection , and Withdrawal of Guidance titled “Revised Recommendations for Reducing the Risk of Zika Virus Transmission by Blood and Blood Components” - FDA has determined Zika virus (ZIKV) is no longer an RTTI under FDA’s regulations because, as discussed further in the guidance, the available evidence demonstrates that ZIKV no longer has sufficient incidence and/or prevalence to affect the potential donor population. Accordingly, FDA withdrew the guidance titled, “Revised Recommendations for Reducing the Risk of Zika Virus Transmission by Blood and Blood Components,” dated July 2018.
- June 3, 2020: FDA cleared the DPP Zika IgM System for detecting Zika virus IgM antibodies. Previously, the test had been authorized only for emergency use under FDA’s EUA authority.
November 25, 2019: Publication - FDA Zika virus reference panel for molecular-based diagnostic devices supports product testing for Emergency Use Authorization and 510(k) submissions - read the full publication in The Journal of Molecular Diagnostics
October 28, 2019: FDA cleared the LIAISON XL Zika Capture IgM Assay II for the presumptive qualitative detection of Zika virus IgM antibodies in human sera collected from individuals meeting the CDC Zika virus clinical and/or epidemiological criteria. Previously, the test had been authorized only for emergency use under FDA’s EUA authority. FDA revoked the EUA for the LIAISON XL Zika Capture IgM Assay II test, initially issued on April 5, 2017.
July 17, 2019: FDA cleared the ADVIA Centaur Zika test. This is the second Zika diagnostic test FDA has allowed to be marketed in the U.S. for detecting Zika virus IgM antibodies. Previously, the test had been authorized only for emergency use under FDA’s EUA authority. FDA revoked the EUA for the ADVIA Centaur Zika test, initially issued on September 18, 2017.
July 3, 2019: In a letter to FDA dated June 18, 2019, Luminex Corporation requested that the EUA for the xMAP MultiFLEX Zika RNA Assay issued on August 4, 2016, and amended on January 7, 2017, and May 19, 2017, be withdrawn. Luminex has decided to discontinue manufacture of the product and there is no remaining viable inventory of the xMAP MultiFLEX Zika RNA Assay. As a result, this product will no longer be marketed, and these circumstances make revocation appropriate to protect the public health or safety. Accordingly, on July 3, 2019, FDA revoked the EUA for xMAP MultiFLEX Zika RNA Assay, pursuant to section 564(g)(2) of the Act. As of July 3, 2019, the xMAP MultiFLEX Zika RNA Assay that was authorized by FDA for use by clinical laboratories for the qualitative detection of RNA from Zika virus is no longer authorized by FDA.
May 23, 2019: FDA authorizes marketing of first diagnostic test for detecting Zika virus antibodies - FDA authorized marketing (PDF, 175 KB) of the ZIKV Detect 2.0 IgM Capture ELISA to detect Zika virus immunoglobulin (IgM) antibodies in human blood. FDA reviewed data for the ZIKV Detect 2.0 IgM Capture ELISA test through the De Novo premarket review pathway. Also see Emergency Use Authorization below
April 18, 2019: EUA amendment - In response to Siemens Healthcare Diagnostic Inc.’s request, FDA concurred (PDF, 137 KB) with the request to modify the ADVIA Centaur Zika test to include surfactant in the ADVIA Centaur Zika IgM assay reagent buffers and the related updates of the Instructions for Use (PDF, 2.8 MB). For more information, see Emergency Use Authorizations (Devices)
February 28, 2019: Important Information for Human Cell, Tissue, and Cellular and Tissue-Based Product (HCT/P) Establishments Regarding Zika Virus Transmission Risk in the World [ARCHIVED] - CDC has changed information on its Blood and Tissue Safety webpage used to communicate epidemiological information about Zika virus (ZIKV) to the blood and tissue collection community. The webpage includes a world map of areas with risk of Zika for other countries and territories outside of U.S. states. A new process has been developed to indicate risk for these areas that assigns one of four categories. FDA considers countries and territories outside the U.S. states categorized as “Red” (current outbreak) or “Purple” (any prior or current reports of mosquito-borne Zika transmission) as areas with increased risk of ZIKV transmission.
For updates by date before 2019, please visit our archive .
Safety of the Blood Supply
FDA is responsible for regulatory oversight of the U.S. blood supply. FDA works closely with other parts of the Public Health Service (PHS) to establish blood standards, and to identify and respond to potential threats to blood safety or supply.
Zika updates - safety of the blood supply
- On February 28, 2019, FDA published a web page: Important Information for Human Cell, Tissue, and Cellular and Tissue-Based Product (HCT/P) Establishments Regarding Zika Virus Transmission Risk in the World (archived) - CDC has changed information on its Blood and Tissue Safety webpage used to communicate epidemiological information about ZIKV to the blood and tissue collection community. The webpage includes a world map of areas with risk of Zika for other countries and territories outside of U.S. states. A new process has been developed to indicate risk for these areas that assigns one of four categories. FDA considers countries and territories outside the U.S. states categorized as “Red” (current outbreak) or “Purple” (any prior or current reports of mosquito-borne Zika transmission) as areas with increased risk of ZIKV transmission.
Revised guidance - On July 6, 2018, FDA announced the availability of a revised final guidance: Revised Recommendations for Reducing the Risk of Zika Virus Transmission by Blood and Blood Components . This revised guidance replaces the August 2016 guidance, which recommended universal nucleic acid testing for Zika virus of individual units of blood donated in the U.S. states and territories. The revised guidance explains that, in order to comply with applicable testing regulations, blood establishments must continue to test all donated Whole Blood and blood components for Zika virus using a nucleic acid test. The revised guidance explains the basis for the FDA’s determination that pooled testing of donations using a screening test licensed for such use by the FDA is a sufficient method for complying with these regulations and effectively reducing the risk of Zika Virus transmission, unless there is an increased risk of local mosquito-borne transmission of Zika virus in a specific geographic area that would trigger individual donation testing in that location. Alternatively, blood establishments may use an FDA-approved pathogen-reduction device for plasma and certain platelet products. ( Federal Register notice ) Also see: FDA announces revised guidance on the testing of donated blood and blood components for Zika virus
Revised guidance - On May 2, 2018, FDA issued revised guidance for establishments that make donor eligibility determinations for donors of human cells, tissues, and cellular and tissue-based products (HCT/Ps): Donor Screening Recommendations to Reduce the Risk of Transmission of Zika Virus by Human Cells, Tissues, and Cellular and Tissue-Based Products; Guidance for Industry (PDF, 86 KB). This guidance updates information in the March 2016 guidance by: This update supports the continuation of recommendations to screen living donors of HCT/Ps for risks of infection with ZIKV based on geographic areas with risk.
This update supports the continuation of recommendations to screen living donors of HCT/Ps for risks of infection with ZIKV based on geographic areas with risk. Previously, on March 1, 2016, as an additional safety measure against the emerging Zika virus outbreak, FDA issued this guidance as a part of ongoing efforts to protect HCT/Ps and blood products from Zika virus transmission. Read the news release
providing findings from more recent epidemiological studies including impact on public health;
reporting new data that informs the potential for transmission of ZIKV;
discussing the current status of availability of ZIKV tests;
updating sexual contact risk factors;
updating when an area is considered to have an increased risk for ZIKV transmission; and,
providing additional scientific references.
On July 5, 2018, FDA approved the Procleix Zika Virus Assay, manufactured by Grifols Diagnostics Solutions, Inc. The Procleix Zika Virus Assay is a qualitative nucleic acid test for the detection of Zika virus RNA in individual plasma specimens obtained from volunteer donors of whole blood and blood components for transfusion. It is also intended for use in testing plasma or serum specimens to screen other living donors of organs and human cells, tissues, and cellular and tissue-based products (HCT/Ps), and in testing blood specimens to screen cadaveric donors. The assay is intended for use in testing individual donor samples. It is also intended for use in testing pools of human plasma composed of equal aliquots of not more than 16 individual specimens from volunteer donors of whole blood components. It is not intended for use as an aid in the diagnosis of Zika virus infection. For more information see the approval letter (PDF, 41.2 KB) and Safety of the Blood Supply below
On October 5, 2017, FDA approved the first test for screening Zika virus in blood donations . FDA approved the cobas Zika test, a qualitative nucleic acid test for the detection of Zika virus RNA in individual plasma specimens obtained from volunteer donors of whole blood and blood components, and from living organ donors. It is intended for use by blood collection establishments to detect Zika virus in blood donations, not for the individual diagnosis of Zika virus infection. Before October 5, 2017, several blood collection establishments used the cobas Zika test under IND in order to follow the recommendations in the FDA’s 2016 guidance document. The data collected from this testing, and from additional studies performed by the manufacturer, demonstrated that the cobas Zika test is an effective test to screen blood donors for Zika virus infection. The test’s clinical specificity was evaluated by testing individual samples from blood donations at five external laboratory sites, resulting in clinical specificity of more than 99 percent. The cobas Zika test is intended for use on the fully automated cobas 6800 and cobas 8800 systems. The cobas Zika test, cobas 6800, and cobas 8800 systems are manufactured by Roche Molecular Systems, Inc. Previously, on March 30, 2016, FDA announced the availability of an investigational test to screen blood donations for Zika virus. The screening test may be used under an investigational new drug application (IND) for screening donated blood in areas with active mosquito-borne transmission of Zika virus. Once screening of blood donations for Zika virus using the investigational test begins, blood establishments in Puerto Rico may resume collecting donations of Whole Blood and blood components.
FDA continues to work with public health authorities in territories with confirmed Zika virus to take rapid and appropriate steps to help ensure safe blood is available. Prior to the revised guidance issued on August 26, 2016, FDA took steps to protect the blood supply in areas with confirmed Zika virus transmission.
On March 5, 2016, the first batch of blood products arrived in Puerto Rico in response to HHS efforts to arrange and fund shipment of blood from the continental United States to Puerto Rico to ensure an adequate supply of safe blood for island residents. The Commonwealth of Puerto Rico was the first U.S. territory to experience active mosquito-borne Zika transmission.of an investigational test to screen blood donations for Zika virus. The screening test may be used under an investigational new drug application (IND) for screening donated blood in areas with active mosquito-borne transmission of Zika virus. Once screening of blood donations for Zika virus using the investigational test begins, blood establishments in Puerto Rico may resume collecting donations of Whole Blood and blood components.
On March 13, 2017, the CDC announced that based on a retrospective analysis of Zika virus (ZIKV) infections they identified a potential increased risk to blood and tissue safety, including semen, in Florida’s Miami-Dade, Palm Beach, and Broward counties dating back to June 15, 2016. While Miami-Dade County is the only part of Florida currently (July 29, 2016 to present) designated by CDC as an area of active ZIKV transmission for the purposes of blood and tissue safety intervention, people in this part of Florida regularly travel within and between these three counties and may not recognize that they have been in an area of active ZIKV transmission. This information has been added to CDC’s webpage used to communicate epidemiological information about ZIKV to the blood and tissue collection community.
The potential increased risk to blood and tissue safety, and particularly to semen, in this area due to CDC’s announcement is considered to be very low. However, as a precaution, the Food and Drug Administration is informing establishments that collect tissues (i.e., human cell, tissues, and cellular and tissue-based products – HCT/Ps) and blood components of the potential increased risk, so they may consider whether and how this new information impacts their practices.
Also see the FDA’s communication to tissue establishments: Important Information for Human Cell, Tissue and Cellular and Tissue-based Product (HCT/P) Establishments Regarding Zika Virus
Emergency Use Authorization
FDA stands ready to use our authorities to the fullest extent to help facilitate the development and availability of products for Zika virus. Under the FDA’s Emergency Use Authorization (EUA) mechanism, the agency can enable the use of an unapproved medical product, or the unapproved use of an approved medical product, during emergencies, when, among other circumstances, there are no adequate approved, and available alternatives. An EUA is an important mechanism that allows broader access to available medical products under specific circumstances.
Zika EUA information
- While many people with Zika virus infection experience no symptoms, the virus can pose potentially serious risks to the public health. Access to a diagnostic test that can identify patients with Zika virus infections is critical to supporting response efforts and expanding domestic readiness. Potential links between Zika virus infection and neurological complications (i.e., Guillain-Barré Syndrome), as well as microcephaly and other poor pregnancy outcomes associated with Zika virus infection during pregnancy, have also increased the importance of having a diagnostic test available for Zika virus. As there are no commercially available diagnostic tests cleared or approved by the FDA for the detection of Zika virus infection, it was determined that an EUA is crucial to ensure timely access to a diagnostic tool.
- An EUA is a tool that FDA can use to allow the use of certain medical products for emergencies based on scientific data. The U.S. Secretary of Health and Human Services (HHS) has declared that circumstances exist to allow the emergency use of authorized diagnostic tests for Zika virus infection.
- Draft EUA review templates for Zika are available to product sponsors/manufacturers by email request to: [email protected]
Zika diagnostic tests currently authorized under EUA
Performance characteristics of zika virus diagnostic tests.
FDA has posted tables detailing performance characteristics of Zika virus diagnostic tests (assays) currently available for use under EUA. The tables include information about analytical sensitivity, along with other performance characteristics determined during EUA evaluation. (May 3, 2018)
- Table 1: Molecular ZIKV EUA Assays - Performance Characteristics (PDF, 200 KB)
- Table 2: Molecular ZIKV EUA Assays - Key Characteristics (PDF, 247 KB)
Tests currently authorized under EUA are listed on the CDRH page, Emergency Use Authorizations for Medical Devices .
For a list of FDA-authorized diagnostic tests for detecting Zika virus antibodies, see Diagnostics above.
Nucleic acid testing-based assays (molecular tests) - detect genetic material in samples of bodily fluids, such as serum and urine, to diagnose active Zika infection
Serological assays - detect antibodies against Zika virus in the blood, to assess whether individuals who may have recently been exposed to Zika have actually been infected
Also see the August 17, 2017 press release: FDA provides new tools for the development and proper evaluation of tests for detecting Zika virus infection - As an additional measure in the fight against Zika virus, FDA made available a panel of human plasma samples to aid in the regulatory evaluation of serological tests, to help ensure that tests to detect recent Zika infection are accurate and reliable, and to help test manufacturers know if their tests differentiate between infections with Zika virus or other flaviviruses such as Dengue, and West Nile viruses, which all have similar antibodies. More: Zika Virus Reference Materials and FDA Zika virus reference panel for molecular-based diagnostic devices supports product testing for Emergency Use Authorization and 510(k) submissions
Investigational Products
Medical Products | Genetically Engineered Mosquitoes
Medical Products (Vaccines, Therapeutics, Diagnostics)
Vaccines and therapeutics: There are no FDA-approved vaccines or treatments for Zika at this time. Several investigational vaccines are under development, including early human clinical trials . FDA is prepared to evaluate the safety and efficacy of any investigational vaccines and therapeutics that might be developed to help mitigate this outbreak.
Diagnostics: For a list of FDA-authorized diagnostic tests for detecting Zika virus antibodies, see Diagnostics above.
FDA encourages commercial diagnostic developers and researchers developing laboratory developed tests for Zika virus to submit an EUA request or consider pursuing a premarket submission. FDA will work interactively with developers to support such requests. See Zika Virus Diagnostic Development for information on FDA support for Zika virus diagnostic development and Emergency Use Authorization for information about Zika virus diagnostics available under EUA.
To help Zika diagnostic manufacturers assess traceability of their tests (a requirement for Emergency Use Authorization), FDA has created the FDA Zika Virus Reference Materials for NAT-based IVD devices , available upon request to Zika device developers who have a pre-EUA submission with the agency and have established the analytical and clinical performance of their assay. In July 2017, FDA also made available a panel of human plasma samples to aid in the regulatory evaluation of serological tests to detect recent Zika virus infection. Developers planning a future premarket submission will have priority to receive the panel of human plasma samples, considering the grant of a De Novo classification request for the ZIKV Detect 2.0 IgM Capture ELISA on May 23, 2019. View an infographic about the FDA Zika Virus Reference Materials (PDF, 120 KB)
Genetically engineered mosquitoes related to Zika response
Final guidance - October 4, 2017: FDA Issues Final Guidance Clarifying FDA and EPA Jurisdiction over Mosquito-Related Products [ARCHIVED] - The final Guidance for Industry #236 – Clarification of FDA and EPA Jurisdiction over Mosquito-Related Products (PDF, 85 KB) – clarifies that mosquito-related products intended to function as pesticides by preventing, destroying, repelling, or mitigating mosquitoes for population control purposes, and that are not intended to cure, mitigate, treat, or prevent a disease are not “drugs” under the Federal Food, Drug, & Cosmetic Act, and will be regulated by the EPA under the Federal Insecticide, Fungicide, and Rodenticide Act. The FDA will continue to have jurisdiction over mosquito-related products that are intended to prevent, treat, mitigate, or cure a disease (including by an intent to reduce the level, replication, or transmissibility of a pathogen in mosquitoes). ( Federal Register notice )
The Zika virus outbreak highlights the importance that novel vector control measures may play in protecting the public health. Reviewing the use of innovative strategies to help suppress the population of virus-carrying mosquitoes is one of many activities in which FDA is engaged to help mitigate the threat of vector-borne epidemics, such as Zika.
FDA’s Center for Veterinary Medicine reviewed information in an Investigational New Animal Drug (INAD) file from Oxitec, Ltd., regarding the company’s genetically engineered line of the mosquito Aedes aegypti (OX513A), with the intent of suppressing the population of that mosquito at the release site(s). Ae. aegypti is known to transmit the debilitating human virus-caused diseases Zika, dengue, yellow fever, and chikungunya. More: Oxitec Mosquito
On March 11, 2016, in compliance with FDA regulations, FDA released for public comment a draft environmental assessment (EA) (PDF, 33 MB) submitted by Oxitec, Ltd., that assesses the potential environmental impacts of a field trial of the company’s genetically engineered (GE) Aedes aegypti mosquitoes (OX513A) in Key Haven, Florida. Ae. aegypti is known to transmit potentially debilitating human viral diseases, including Zika, dengue, yellow fever and chikungunya. The FDA also released a preliminary finding of no significant impact (FONSI) (PDF, 148 KB) that agrees with the draft EA’s conclusion that the field trial of such GE mosquitoes will not result in significant impacts on the environment.
The goal of the proposed field trial is to determine whether released Oxitec GE mosquitoes will mate with local wild-type Aedes aegypti and suppress their population at the release site. The proposed study is not seeking to evaluate whether release of Oxitec’s GE mosquitoes will reduce Zika virus transmission. Oxitec’s mosquitoes are one possible approach that could be incorporated into an integrated program to help mitigate the threat of vector-borne epidemics; however, it is too early to say with any certainty whether such an approach would be successful.
The public comment period for the draft Environmental Assessment and preliminary Finding of No Significant Impact concerning investigational use of Oxitec OX513A mosquitoes closed on May 13, 2016. Because this is a first of its kind application, FDA understands how important the public comment period process is.
August 5, 2016: FDA Releases Final Environmental Assessment for Genetically Engineered Mosquito [ARCHIVED] - FDA has completed the environmental review for a proposed field trial to determine whether the release of Oxitec Ltd.’s genetically engineered (GE) mosquitoes (OX513A) will suppress the local Aedes aegypti mosquito population in the release area at Key Haven, Florida. After considering thousands of public comments, FDA has published a final environmental assessment (EA) (PDF, 3 MB) and finding of no significant impact (FONSI) (PDF, 198 KB) that agrees with the EA’s conclusion that the proposed field trial will not have significant impacts on the environment. FDA’s finalization of the EA and FONSI does not mean that Oxitec’s GE mosquitos are approved for commercial use. Oxitec is responsible for ensuring all other local, state, and federal requirements are met before conducting the proposed field trial, and, together with its local partner, the Florida Keys Mosquito Control District, to determine whether and when to begin the proposed field trial in Key Haven, Florida.
January 18, 2017: FDA Requests Comments on Documents Related to Certain Biotechnology and Mosquito-related Products - FDA is requesting public comment on a draft revised guidance (PDF, 200 KB) on the regulation of animals with intentionally altered genomic DNA, including animals produced through the use of genome editing and genetic engineering, and a draft guidance (PDF, 74 KB) that clarifies which mosquito-related products FDA regulates and which such products EPA regulates, regardless of whether these mosquito-related products are developed using biotechnology. Also see Oxitec Mosquito ; Q&A on FDA Regulation of Intentionally Altered Genomic DNA in Animals ; and FDA Voice: FDA’s Science-based Approach to Genome Edited Products
April 12, 2017: FDA is extending the comment period to continue seeking public input on draft revised guidance for industry #187 - Regulation of Intentionally Altered Genomic DNA in Animals (PDF, 200 KB). The FDA is taking this action in response to requests for additional time to submit comments. The comment period will now close on June 19, 2017 . Also see: FDA Requests Comments on Documents Related to Certain Biotechnology and Mosquito-related Products and Q&A on FDA Regulation of Intentionally Altered Genomic DNA in Animals
October 4, 2017: FDA Issues Final Guidance Clarifying FDA and EPA Jurisdiction over Mosquito-Related Products - The final Guidance for Industry #236 – Clarification of FDA and EPA Jurisdiction over Mosquito-Related Products (PDF, 85 KB) (additional details above)
Fraudulent Products
- Unfortunately, during outbreak situations, fraudulent products claiming to prevent, treat or cure a disease almost always appear. FDA monitors for fraudulent products and false product claims related to the Zika virus and takes appropriate action to protect consumers. Consumers who have seen these fraudulent products or false claims are encouraged to report them to the FDA.
Using Insect Repellents Safely
- All insect repellents, including products combined with sunscreen, should be used according to instructions on the label.
- Use insect repellents that contain active ingredients registered by the Environmental Protection Agency (EPA) for use on skin and clothing. EPA registration of insect repellent active ingredients indicates the materials have been reviewed and approved for human safety and effectiveness when applied according to instructions on the label.
- Don't use insect repellent on babies. Repellent used on older children should contain no more than 10 percent DEET. Oil of eucalyptus products should not be used in children under 3 years.
For a list of events 2019 and earlier, please visit our archive .
More About FDA's Role
FDA is committed to working with the global community as it responds to the Zika virus outbreak. The FDA has a critical role in facilitating the development, and availability of investigational products for use against emerging infectious diseases, such as the Zika virus.
FDA is actively working with our Federal colleagues at the CDC, National Institutes of Health (NIH), and the Biomedical Advanced Research and Development Authority (BARDA), and is prepared to evaluate the safety and efficacy of any investigational vaccines and therapeutics that might be developed to help mitigate this outbreak. The agency is also encouraging development of diagnostic tests that may be useful for identifying the presence of the virus, and is taking steps to help ensure the safety of our nation’s blood supply.
While FDA cannot comment on the development of specific medical products, it’s important to note that every FDA regulatory decision is based on a risk-benefit assessment of scientific data that includes the context of use for the product and the patient population being studied. Approaches that will be able to show whether a product has a favorable risk-benefit profile for its proposed use may require careful planning. This may prove challenging for Zika virus since its symptoms are often mild or nonspecific.
Emergency use: FDA stands ready to use our authorities to the fullest extent to help facilitate the development and availability of products for Zika virus, as we did during the 2014 Ebola epidemic. Under the FDA’s Emergency Use Authorization (EUA) mechanism, the agency can enable the use of an unapproved medical product, or the unapproved use of an approved medical product, during emergencies, when, among other circumstances, there are no adequate approved, and available alternatives. An EUA is an important mechanism that allows broader access to available medical products under specific circumstances.
Blood supply: FDA is responsible for regulatory oversight of the U.S. blood supply. FDA works closely with other parts of the Public Health Service (PHS) to establish blood standards, and to identify and respond to potential threats to blood safety or supply. More: Keeping Blood Transfusions Safe: FDA's Multi-layered Protections for Donated Blood
Translations
Español Português
Note: Spanish and Portuguese translations of this page are archived, and were last updated on the date listed at the bottom of the archived page.
Contact FDA
General Info/Consumers 1-888-INFO-FDA / (1-888-463-6332)
Report a Fraudulent Zika Product Report form and instructions
Press Office of Media Affairs [email protected] 301-796-4540
Clinicians Emergency Investigational New Drug (EIND) Applications for Antiviral Products
Diagnostic Product Sponsors/Manufacturers - EUA Templates Draft EUA review templates for Zika are available by email request to: [email protected]
Related Links
- Zika Virus Information from CDC
- Zika Virus Health Information Resources (National Library of Medicine)
- How to Avoid Bug Bites (CDC)
- About Emergency Use Authorization
- The FDA's Drug Review Process: Ensuring Drugs Are Safe and Effective
Resources for Healthcare Providers
- Zika Information for Health Care Providers (CDC)
- Zika Training for Health Care Providers (CDC)
- When to test for Zika virus (CDC) (PDF, 332 KB)
- Promoting Stress Management for Pregnant Women during the Zika Virus Disease Outbreak: A Resource for Healthcare Providers, and Planning Resources (HHS)
- Guidance for U.S. Laboratories Testing for Zika Virus Infection (CDC)
Sign up to receive email alerts on emergency preparedness and response topics from FDA, including medical countermeasures and emerging infectious diseases.
Whatever Happened to Zika?
The problem with jumping between emergencies

Produced by ElevenLabs and News Over Audio (NOA) using AI narration.
In 2015, a horror movie came to life. The mosquitoes that swarm almost all tropical climates began infecting people with a strange new virus. In most, Zika caused no symptoms, or a mild rash and fever. But if it happened to infect a pregnant woman, her baby could be born with severe birth defects. Zika dramatically increased the risk of a condition called microcephaly, or a clinically small head. Over the following years, about 4 to 9 percent of infected pregnant women gave birth to babies with permanent brain damage.
Suddenly, pregnant women in America and elsewhere were told not to travel to the Caribbean and South America. Expecting mothers in Miami, where local mosquitoes were transmitting the virus, stayed inside all summer long. Today, thousands of Brazilian families struggle to care for profoundly disabled 8-year-olds , “their limbs rigid, their mouths slack, many with foreheads that sloped sharply back above their dark eyes,” as The New York Times described in 2022.
Then, as quickly as it appeared, Zika vanished from global awareness. In 2016, most major news sites, including this one, largely stopped covering the disease regularly. Despite the absence of a treatment or vaccine, the world’s attention moved on.
Read: The answer to Zika may be more mosquitos
There are good reasons for this: Zika cases dropped precipitously after 2016. And just a few years later, COVID ravaged the planet, giving us all something new to worry about. But that doesn’t mean Zika is gone. The disease is still out there, infecting people every day. There is still no Zika vaccine, and experts say another outbreak is likely before too long. In this way, Zika reflects a typical epidemic cycle—an emergent crisis, followed by a brief influx of resources, followed by rich countries’ long and fateful forgetting. “A lot of people have forgotten about Zika,” says Anna Durbin, a professor of global health at Johns Hopkins. “They think because we don’t see a big outbreak that it’s not there, but it’s definitely there. And it can be devastating for children born with congenital Zika syndrome.”
By 2017, Zika had burned through entire cities. Some experts estimate that the virus infected half the residents of Recife, a Northeastern Brazilian city and the outbreak’s epicenter. This swift onslaught was tragic, but it had an upside: Countries in the Caribbean and the Americas quickly achieved herd immunity, essentially starving the virus of new hosts. Cases fell off rapidly—in 2018, about 30,000 Zika cases were reported in the Americas, a region that spans between Argentina and Canada. Compare that with nearly 650,000 in 2016 .
But despite this overall improved picture, the virus continues to circulate. In 2022, the Americas saw 40,528 cases of Zika. Brazil had the greatest number of cases that year, at more than 34,000, but Belize had the highest incidence per capita. As of early December, 31,780 cases were reported in the Americas in 2023. Microcephaly is far less prevalent, but it, too, is still occurring: Brazil saw 163 cases of Zika-linked microcephaly in 2022, according to the Pan American Health Organization, down from 2,033 in 2016 . And growing evidence indicates that Zika can cause brain damage beyond microcephaly, including calcification in the brain and other, less noticeable issues. These effects are even less well tracked.
What’s more, these numbers are “probably just the tip of the iceberg,” says Albert Ko, a professor at the Yale School of Public Health. Up to 80 percent of people infected with Zika experience no symptoms and don’t get tested, and therefore would not be included in these case counts. There might be much more Zika circulating, and nobody is aware of it.
Tests for Zika are expensive and not readily obtainable, and the countries most affected by the virus have cash-strapped health systems. During the coronavirus pandemic, countries with limited lab resources shifted to diagnosing COVID, and Zika fell by the wayside. “The big problem is that many countries are not reporting Zika, or they’re not systematically testing for Zika,” Ko told me.
Herd immunity provided a temporary reprieve, but it also created a new problem. A lower incidence of Zika meant less commercial interest in making a vaccine, because the market for a Zika vaccine would by definition be smaller. Vaccine companies also struggled to find populations in which to test a vaccine, because too few people now had confirmed Zika infections. “The general momentum that was behind the development of a Zika vaccine ground to a halt,” says Jennifer Nuzzo, the director of the Pandemic Center at Brown University.
For now, this holding pattern might be acceptable, but it won’t be for long. Scientists don’t know how long Zika immunity lasts, and as time goes on, more and more people are being born who are immunologically naive: They’ve never been exposed to the virus before. Various experts predicted to me that five, 10, or 20 more years might pass without much Zika, after which we’ll see a smaller yet still sizable new outbreak.
When that happens, it will affect, primarily, poor women who live in the global South. In Recife, 97 percent of microcephaly cases occurred in children of women of low or medium socioeconomic status, according to Ernesto Marques, an infectious-disease professor at the University of Pittsburgh who has tracked Zika closely in Brazil. Poor women bore the brunt of Zika perhaps because they were more likely to live in areas without air-conditioning or good sanitation, and thus had greater exposure to mosquitoes. These women face both the ongoing stress of a potential Zika infection and the looming specter of another big flare-up in the community.
But the unresolved risk of Zika also threatens all citizens of these countries. Many nations most heavily affected by Zika rely on tourism to power their economy. Brazil is one of the largest economies in the world and attracts millions of visitors every year. Puerto Rico, which was hit hard by Zika, is part of the U.S., and thousands of women on the American mainland might travel back and forth to see family. Most experts I talked with said the risk was small for female travelers, because they would likely stay in hotels with air-conditioning and at least some mosquito control. But there’s no such thing as no risk.
Because Zika is currently circulating, but at low levels, official advice to pregnant women considering travel to these countries is ambiguous. Regarding almost every country in the Americas, the CDC warns that it has reported “past or current Zika virus transmission” but that “we do not have accurate information on the current level of risk.” It suggests that pregnant women and those who are considering getting pregnant “work with their health care providers to carefully consider the risks and possible consequences of travel to areas with risk of Zika.” In other words, caveat traveler.
When I asked the Pan American Health Organization, a spokesperson recommended that pregnant women who go to these places “take comprehensive measures to prevent mosquito bites, such as the use of insect repellents, the use of clothing (preferably light-colored) that covers most of the body, the use of bed nets and mosquito screens for windows and doors to prevent mosquitoes from entering the houses.” But who is going to wear a full-coverage outfit in a tropical climate? Asking every woman who is pregnant or even thinking about getting pregnant to apply these precautions consistently does not seem like a viable public-health strategy.
To be fair, some scientists are working to prepare for the next Zika wave; several met in the U.K. in December to discuss Zika research. Some organizations are working on a plan that would send mosquitoes infected with a bacterium that inhibits Zika into affected countries, in the hope that, over time, the infected mosquitoes would reduce Zika transmission. Several companies, including Moderna, maker of one of the COVID vaccines, are now working on a Zika vaccine. “But I think there’s still a lot of questions about what would the demand be? What is the target population? How are we going to fund that?” Durbin told me.
Experts I interviewed seemed frustrated that the world is not better prepared for when Zika strikes again. Multiple companies spun up a COVID vaccine in about a year because the U.S. government guaranteed that they’d get paid to do so. Zika emerged in Brazil eight years ago, and no similar guarantee seems forthcoming.
Read: Will winter kill Zika?
Instead, the political leaders of wealthy countries seem to jump from emergency to emergency, never quite internalizing the lessons from the most recent pandemic. Even before COVID hit, testing for Zika was difficult and spotty, including in rich countries like the U.S. But the American health-care system never learned from that failure: Testing for COVID was difficult and spotty in the early days of the coronavirus pandemic, and then, in 2022, testing for Mpox was difficult and spott y too.
It’s understandable that the U.S. government isn’t treating Zika as an emergency now that the crisis has subsided. But, as Nuzzo put it to me, “you don’t just shut down the fire station because you put the fire out.” If you do, you risk going up in flames.
About the Author

More Stories
The Well-Off People Who Can’t Spend Money
Why Can’t I Just Rent a House?

Zika in India: CDC issues travel advisory for Maharashtra state

This week, the US Centers for Disease Control and Prevention (CDC) issued a warning for travelers to the state of Maharashtra, in the western peninsular region of India, due to a Zika virus outbreak.
A total of 97 positive Zika virus cases have been reported in Maharashtra as of August 9, to include 45 cases in pregnant women.
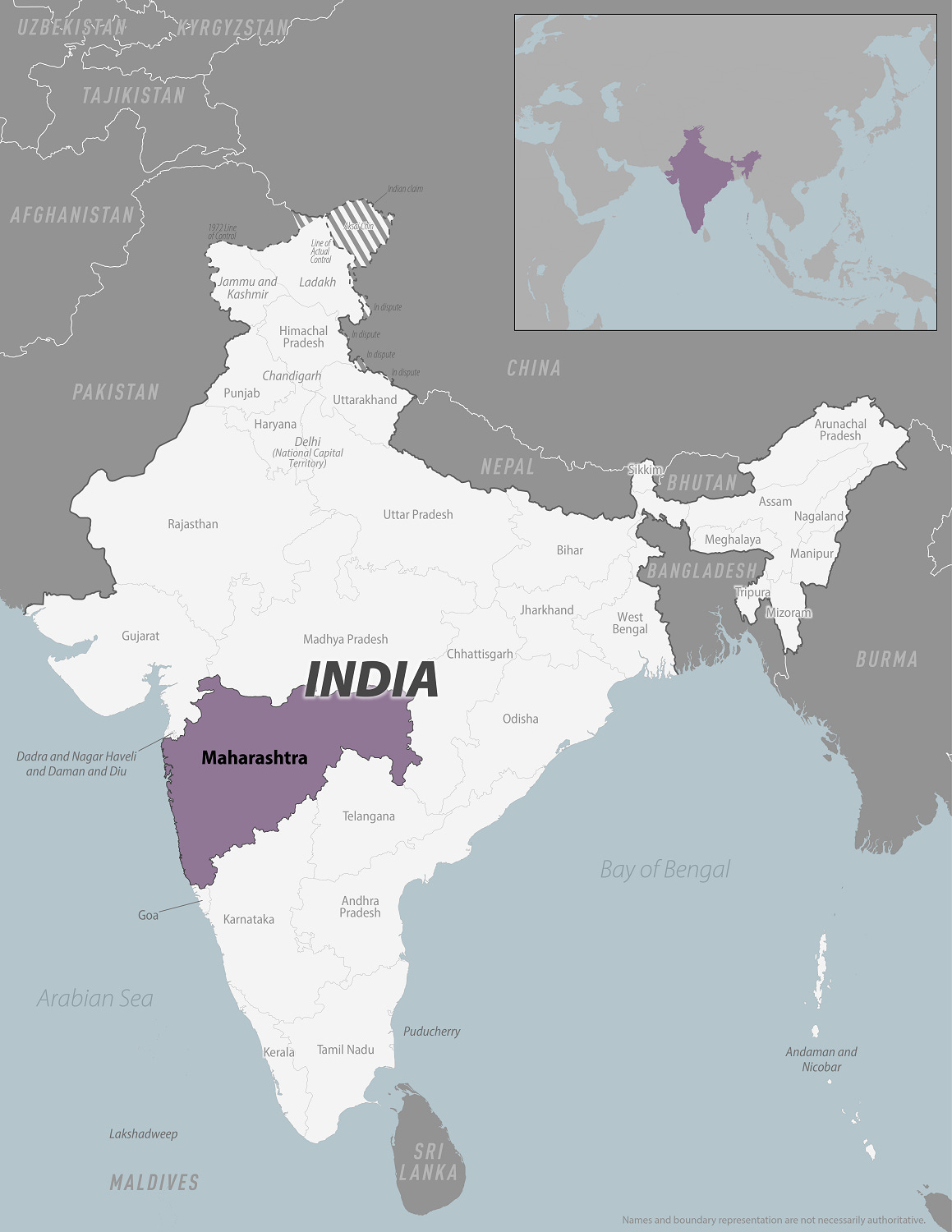
The city of Pune accounts for the most cases with 75, or 77 percent of all cases.
Health officials in the state said they are closely monitoring pregnant women with positive Zika virus cases.
Health officials in the US recommend travelers to Maharashtra should take steps to prevent mosquito bites and sexual transmission of Zika virus during and after travel.
If you are pregnant or planning pregnancy, talk to your doctor about your risk of Zika virus infection, the possible health effects of Zika virus infection during pregnancy, and ways to protect yourself.
If you are pregnant, you should avoid travel to Maharashtra. If travel is unavoidable, you should strictly follow Zika prevention recommendations.
If you are planning pregnancy, you should delay pregnancy following travel based on the timeframes to prevent sexual transmission .
Travelers to Maharashtra should seek medical care immediately if they develop fever, rash, headache, joint or muscle pain, or red eyes during or after travel.
The first Zika virus infection case in Maharashtra was reported in July 2021 in a 50-year-old woman residing in Belsar village of Purandar tehsil in Pune district. The state reported 27 cases of Zika virus infection in 2021, followed by three cases in 2022, and 15 cases in 2023.
Subscribe to Outbreak News TV on YouTube
Zika is a disease caused by infection with Zika virus and typically occurs in tropical and subtropical areas in parts of Africa, the Americas, Asia, and the Western Pacific.
Zika virus is most commonly spread to people by the bite of an infected Aedes species mosquito. It can also be spread through sex from a person who is infected with Zika virus to their sexual partner. Zika virus can be passed from a pregnant person to their fetus.
Many people infected with Zika virus will not have symptoms or will only have mild symptoms. The most common symptoms are fever, rash, headache, joint and muscle pain, and red eyes. Infection during pregnancy can cause certain birth defects.
There is currently no vaccine to prevent or medicine to treat Zika.
Thanks for reading Outbreak News Today! Subscribe for free to receive new posts and support my work.
Ready for more?

Current or past transmission but no Zika outbreak
No Zika-spreading mosquitoes
Zika mosquitoes present, but no reported cases
Zika Virus Countries 2024
Zika is a virus spread by Aedes mosquitoes ( Ae. aegypti and Ae. albopictus ), sexual contact, or the birth process, that has no vaccine or treatment. Although usually harmless, Zika can on rare occasions cause serious health issues , such as swelling of the brain, blood disorders, or Guillain-Barré syndrome. Zika is a particular concern for pregnant women, as contracting the disease during pregnancy can cause the child to be born with significant birth defects , including microcephaly (an abnormally small head).
The risk of contracting Zika today is minimal, as eradication efforts aimed at containing its spread have been effective. Every country’s Zika status falls into one of four categories:
- Outbreak — The most severe status, this designation is given only to countries that are in the midst of a notable Zika outbreak. As of April 2022, it is not currently in effect anywhere in the world).
- Current or past cases exist, but no current outbreak — This label is used to indicate a country that has recorded at least one case of Zika at some point, confirming that contracting Zika is a possibility, but which is not currently dealing with a Zika outbreak. Still, travelers who are pregnant or trying to become pregnant and plan to visit any of these countries may wish to consult their doctor about how to prevent Zika.
- Zika-carrying mosquitoes present but no reported cases — This category includes countries in which Aedes mosquitos exist, but which have no recorded cases of Zika.
- No Zika-carrying mosquitoes present — The lowest threat level possible. Aedes mosquitoes are not present in countries with this designation, so the chances of contracting Zika are virtually non-existent.
Zika in the modern era
As of April 2022, there has been one significant outbreak in India in November of 2021 . Health experts have warned that a new outbreak could happen at any time , requiring only a single mutation to generate a new variant of the virus.
- Data current as of December 2023.
Download Table Data
Enter your email below, and you'll receive this table's data in your inbox momentarily.
Which countries have the Zika virus?
Frequently asked questions.
- Zika Travel Information - CDC
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- My Account Login
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Open access
- Published: 28 August 2024
Ad26.M.Env ZIKV vaccine protects pregnant rhesus macaques and fetuses against Zika virus infection
- Amanda J. Martinot ORCID: orcid.org/0000-0001-6237-6191 1 , 2 ,
- Freek Cox 3 ,
- Peter Abbink 1 ,
- Jonathan L. Hecht 4 ,
- Roderick Bronson 5 ,
- Erica N. Borducchi 1 ,
- William J. Rinaldi 6 ,
- Melissa J. Ferguson 6 ,
- Rafael A. De La Barrera 7 ,
- Roland Zahn ORCID: orcid.org/0000-0003-2822-6231 3 ,
- Leslie van der Fits 3 &
- Dan H. Barouch ORCID: orcid.org/0000-0001-5127-4659 1 , 8
npj Vaccines volume 9 , Article number: 157 ( 2024 ) Cite this article
1 Altmetric
Metrics details
At the start of the Zika virus (ZIKV) epidemic in 2015, ZIKV spread across South and Central America, and reached parts of the southern United States placing pregnant women at risk for fetal microcephaly, fetal loss, and other adverse pregnancy outcomes associated with congenital ZIKA syndrome (CZS). For this reason, testing of a safe and efficacious ZIKV vaccine remains a global health priority. Here we report that a single immunization with Ad26.M.Env ZIKV vaccine, when administered prior to conception, fully protects pregnant rhesus macaques from ZIKV viral RNA in blood and tissues with no adverse effects in dams and fetuses. Furthermore, vaccination prevents ZIKV distribution to fetal tissues including the brain. ZIKV associated neuropathology was absent in offspring of Ad26.M.Env vaccinated dams, although pathology was limited in fetuses from non-immunized, challenged dams. Vaccine efficacy is associated with induction of ZIKV neutralizing antibodies in pregnant rhesus macaques. These data suggest the feasibility of vaccine prevention of CZS in humans.
Similar content being viewed by others

Protective efficacy of a Zika purified inactivated virus vaccine candidate during pregnancy in marmosets
Maternal vaccination and protective immunity against Zika virus vertical transmission
Efficacy of an inactivated Zika vaccine against virus infection during pregnancy in mice and marmosets
Introduction.
In late 2015, an epidemic of fetal microcephaly in Brazil associated with high levels of circulating Zika virus (ZIKV), led to a global race to develop a ZIKV vaccine to protect women and unborn children from the potential devastating effects of congenital ZIKA syndrome (CZS) 1 , 2 , 3 , 4 . ZIKV, a member of the Flaviviridae family, was first identified in Uganda in 1954, and while sharing a genus with other viruses that cause significant human disease such as dengue, yellow fever, Japanese encephalitis, and West Nile viruses, had only been associated with asymptomatic to mild flu-like symptoms prior to reports of CZS in Brazil in late 2015. Similar to other medically important flaviviruses, ZIKV is primarily acquired by Aedes mosquitos, and can also be spread transplacentally in pregnant women 5 , 6 , in blood transfusions 7 , and through sexual contact 8 . Recently, genetic polymorphisms have been associated with development of CZS 9 .
The World Health Organization (WHO) declared an end to the ZIKV epidemic at the end of 2016 10 , and ZIKV transmission is currently at low levels world-wide, however vaccine development for emergency deployment remains a high priority by the WHO 11 . ZIKV also remains a concern for individuals traveling to endemic areas and for individuals living in areas with continued transmission such as the Caribbean where over 15% of pregnant women continue to test positive for ZIKV 12 . CDC guidance still recommends that pregnant women, partners of pregnant women, or those considering pregnancy to delay travel to areas with ZIKV outbreaks and to consult with medical providers before traveling to ZIKV endemic regions 13 .
Current low levels of ZIKV world-wide limit the establishment of clinical trial sites and enrollment of participants in Phase III efficacy studies 11 , 14 . Ideally a ZIKV vaccine could be deployed in the event of a ZIKV resurgence and administered safely to pregnant women. Preclinical studies in mice and non-human primates (NHP) have shown that induction of neutralizing antibodies by a number of vaccine platforms is effective in preventing ZIKV acquisition 15 , 16 , 17 , 18 ). A number of candidate ZIKV vaccines have completed safety studies in Phase I and II clinical trials 19 , 20 , 21 , 22 . The macaque model has been a useful model for studying dynamics of viral replication and shedding during ZIKV infection 16 , 23 , 24 , 25 , 26 . We and others have shown that the rhesus macaque model consistently reproduces features of ZIKV infection in pregnancy including prolonged ZIKV vRNA in blood and persistence of ZIKV vRNA in lymphoid tissues and the placenta 23 , 26 , 27 , 28 , 29 , 30 , 31 , 32 , 33 . Although fetal microcephaly has not been reported as a fetal outcome of ZIKV infection in monkeys, experimental ZIKV challenge of pregnant non-human primates has recapitulated other adverse outcomes of ZIKV exposure observed in human pregnancy including fetal loss, fetal cerebral calcifications, gliosis, and long-term developmental alterations in infant macaques 30 , 34 , 35 , 36 . As in humans, these events represent a small proportion of overall pregnancy outcomes. While DNA vaccination can protect against ZIKV vRNA in blood, break-through viral replication was seen in a subset of animals 16 and DNA vaccination was only partially protective against vRNA in blood in pregnant macaques 37 . Attempts to treat pregnant monkeys with cocktails of neutralizing antibodies also failed to prevent ZIKV vRNA in blood and adverse fetal outcomes were observed including fetal loss 27 .
Adenoviruses are a wide-spread cause of common upper respiratory tract infections that are typically mild and self-limiting. Adenovirus vectored vaccines are non-replicating yet self-adjuvanting due to their potent induction of innate anti-viral responses that contribute to the induction of broad cellular and humoral immune responses after a single immunization 38 . Ad26 has emerged as a lead Ad vector platform due to low levels of pre-existing immunity and induction of durable neutralizing Ab responses 39 . A common correlate of protection for currently licensed flavivirus vaccines including the yellow fever, dengue, and west nile viruses is the induction of neutralizing antibodies against the envelope (Env) protein 40 . Ad26.M.Env is a monovalent recombinant Ad26-based ZIKV vaccine candidate that encodes for ZIKV membrane (M) protein, lacking the peptide precursor, and envelope (Env) antigens (amino acids 216–794 of the polyprotein) derived from the ZIKV strain BeH815744 that was shown to be immunogenic in animal models 41 ). This construct has also been tested in a human Phase I study (Ad26.ZIKV.001) where it was shown to be well-tolerated and immunogenic 22 . In addition, it was shown to be protective against fetal demise in pregnant interferon alpha/beta receptor knock-out mice 42 ), a highly susceptible model where ZIKV infection leads to high levels of ZIKV plasma vRNA levels and placental ZIKV vRNA and fetal loss 43 . We opted to test this vaccine for safety and efficacy in pregnant rhesus macaques that were ZIKV challenged during the critical equivalent of human first trimester pregnancy. Here we show that Ad26.M.Env prevents peripheral blood ZIKV vRNA and tissue vRNA in pregnant macaques and fetuses with no evidence of ZIKV-associated fetal pathology in rhesus monkeys.
Ad26.M.Env vaccination induced potent anti-ZIKV neutralizing antibodies and prior vaccination did not impact conception in female macaques
Thirteen female macaques were immunized with 10 11 vp of Ad26.M.Env expressing both the ZIKV M protein transmembrane domain without the peptide precursor, and the envelope (Env) antigens (Ad26.M.Env) and were returned to the breeding colony 17 days post-vaccination (Fig. 1a ). Dams were vaccinated a minimum of 2 months and a maximum of 7 months prior to challenge. An additional 13 females were selected that received no vaccination that were placed in the breeding colony at the same time as the vaccine group. No adverse events were reported in either vaccinated or non-vaccinated animals prior to ZIKV challenge. Some animals in both the vaccinated and non-vaccinated groups had reports of weight loss, diarrhea, and trauma that required clinical intervention, but need for clinical intervention was not linked to vaccination or ZIKV challenge. Vaccinated and non-vaccinated females were monitored clinically by complete red blood cell count (CBC). Animals generally maintained normal reference ranges for white blood cell count, red blood cell count, and total lymphocyte counts through-out the study period (Supplementary Fig. 1 ).

a Macaques were vaccinated 17 days prior to introduction into breeding groups, monitored for pregnancy every two weeks and challenged with ZIKV-BR six weeks post-conception. ZIKV-PR neutralizing antibody responses in sera of dams ( n = 9) immunized with 10 11 vp Ad26.M.Env ( b , d ) or non-immunized control animals ( n = 5) ( c ) determined by FRNT. d Colored connective lines represent the neutralization response in time. Neutralizing antibody titers are reported as the log10 of the inverse of the serum dilution that reduce the number of input virus by 50% (IC50). The mean responses per group are indicated with a horizontal line. The dashed line shows the lower limit of detection (LOD) defined as the log10 of one dilution below the start dilution of the samples (0.70 log10). Individual animals of group 1 (Ad26.M.Env immunized) are color coded to represent number of weeks between immunization and challenge; green (10 weeks); purple (12 weeks); blue (14 weeks); gray (18 weeks); red (22 weeks); brown (30 weeks). Black arrow indicates time of vaccination; colored arrows indicate time of challenge matched to inter-immunization and challenge interval color in figure legend. Amino amniocentesis, CSF cerebral spinal fluid, CR colorectal swab, CV cervical swab, PO oropharyngeal swab. b Friedman one-way ANOVA, followed by Dunn’s test for multiple comparisons ( c ) Mann–Whitney test; * p = /< 0.05; ** p = /< 0.01; *** p = < 0.001. Samples below LOD were set to 0.70.
Females in all groups were monitored bi-weekly by ultrasound for pregnancy. Pregnant females in the Ad26.M.Env vaccine and no vaccine groups were infected with 1×10 6 vp of ZIKV via the subcutaneous route 6 weeks post-conception (10–30 weeks post-vaccination based on timing of confirmed pregnancy; Supplementary Table 1 ). Three pregnant non-vaccinated animals were not challenged to serve as normal pregnant controls. Plasma, sera, cerebral spinal fluid, urine, colorectal, cervical, and saliva samples were collected during pregnancy from all dams as indicated (Fig. 1a ).
Neutralizing antibody titers were determined by both Immunospot focus reduction neutralization (FRNT) and by microneutralization assay (MN50 VNA). Before immunization, animals (OBF, 079 and 437) showed very low neutralization titers, whereas the other six animals showed no neutralization titers when assayed with FRNT (Fig. 1b ). Four weeks after immunization, all animals developed a neutralizing response that was maintained or only marginally decreased 8 weeks after immunization and in pre-challenge serum which was obtained 10 to 30 weeks after immunization (Fig. 1b, d ). Four weeks after challenge, all animals of the Ad26.M.Env immunized group had increased ZIKV neutralization titers (Fig. 1b ). Serum of animals of the non-immunized control group were assayed pre- and post- challenge. Pre-challenge, no neutralization titers were detected with FRNT analysis, whereas ZIKV neutralization titers developed after challenge (Fig. 1c ). These FRNT results were confirmed by the MN50 neutralization assay (Supplementary Fig. 2 ). Vaccinated animals (Group 1) had higher neutralizing Ab four weeks post-vaccination compared to non-vaccinated animals ( p = 0.0010, Mann–Whitney test). Post-challenge neutralizing titers were comparable between Ad26.M.Env and non-vaccinated animals (Mann–Whitney test), consistent with minimal anamnestic antibody responses in vaccinated animals and rapid virus neutralization (Fig. 1b, c , Supplementary Fig. 2 ). Next, antibody responses against ZIKV NS1 protein were measured by ELISA. Ad26.M.Env does not contain a NS1 antigen. In accordance, Ad26.M.Env vaccinated or non-vaccinated animals had low (079) or undetectable NS1 binding antibody responses in pre-challenge samples. Four weeks after challenge, all 5 non-vaccinated dams developed high NS1-specific antibody titers (mean titer of 3.69 log10). Eight out of 9 dams that received Ad26.M.Env also developed NS1-specific titers after challenge although the group mean NS1 titer (1.75log10) was approximately 100-fold (2 log10) lower compared to the group mean NS1-titer in the non-immunized animals ( p = 0.005, Mann–Whitney test; Supplementary Fig. 3 ).
Ad26.M.Env vaccination induced anti-Env cellular immune responses in macaques
Env and prM directed cellular immune responses were measured by IFNγ ELISPOT on frozen PBMC’s isolated pre- immunization, post-immunization, pre-challenge, and post-challenge. Ad26.M.Env vaccination resulted in induction of ZIKV specific cellular responses (Fig. 2 ). The geometric mean Env-specific cellular immune responses in the group that received Ad26.M.Env was above the LOD of the assay, determined at 50 SFU per 10 6 PBMCs, at week 4 and 8 after immunization, and at the pre-challenge timepoint (geomean SFU 55.03, 55.92, and 97.41, respectively). The Env-specific cellular immune responses after immunization were higher when compared to the Env-specific cellular immune responses pre-immunization, or in non-immunized animals which were both below the limit of detection (Fig. 2a, b ). Vaccinated animals had significantly higher anti-Env cellular immune responses pre-challenge ( p = 0.0106) and post-challenge ( p = 0.0470) compared to non-vaccinated animals (Mann–Whitney test; with data points below the LOD of 50 SFU set on LOD). The prM-specific cellular responses were generally low and geometric mean responses do not exceed the cut-off of 50 SFU per 10 6 PBMCs (Fig. 2c, d ). Notably, Env and prM cellular responses did not increase after challenge as compared to the pre-challenge timepoint indicating a lack of anamnestic cellular responses in Ad26.M.Env vaccinated animals (Fig. 2a, c ).

IFNγ ELISPOT responses in PBMCs of dams ( n = 9) immunized with 10 11 vp Ad26.M.Env ( a , c ) or non-immunized control animals ( n = 5) ( b , d ). a , b Env-specific responses and ( c , d ) PrM specific responses are shown. The geometric mean response per group is indicated with a horizontal line. Responses above 50 SFU per 10 6 PBMCs indicated by the dotted line are considered positive. Individual animals of group 1 (Ad26.M.Env immunized) are color coded to represent number of weeks between immunization and challenge; green (10wk); purple (12 weeks); blue (14 weeks); gray (18 weeks); red (22 weeks); brown (30 weeks). a Friedman one-way ANOVA, followed by Dunn’s test for multiple comparisons; * p = /< 0.05; ** p = /< 0.01; *** p = < 0.001. Samples below LOD were set to 50.
Ad26.M.Env vaccinated pregnant females were completely protected against ZIKV vRNA in blood and tissues
Pregnant females in the Group 1 (Ad26.M.Env vaccinated) and Group 2 (non-vaccinated) were infected with 1x10 3 PFU (1×10 6 vp) Zika virus from the 2015 Brazilian epidemic at 6 weeks post-conception (being 10 to 30 weeks post vaccination). Vaccinated dams had no detectable virus in plasma post-ZIKV challenge even though animals were challenged from 10 to 30 weeks after vaccination, depending on the timepoint of conceiving (Fig. 3a ). In contrast, non-vaccinated, challenged pregnant macaques all had detectable viral load, with a mean peak vRNA of 5.5 log10 on day 7 post-challenge (Fig. 3b ) consistent with peak vRNA reported for ZIKV infected non-pregnant and pregnant macaques 23 , 25 , 26 , 28 , 32 , 36 , 37 , 44 , 45 . On average, non-vaccinated, challenged animals had detectable virus for 42 days with a range of 7 to 56 days consistent with previous reports that pregnancy prolongs ZIKV viremia in rhesus monkeys. All colorectal, vaginal, saliva, and amniocentesis samples were negative for ZIKV vRNA for all ZIKV-challenged dams irrespective of vaccination status.
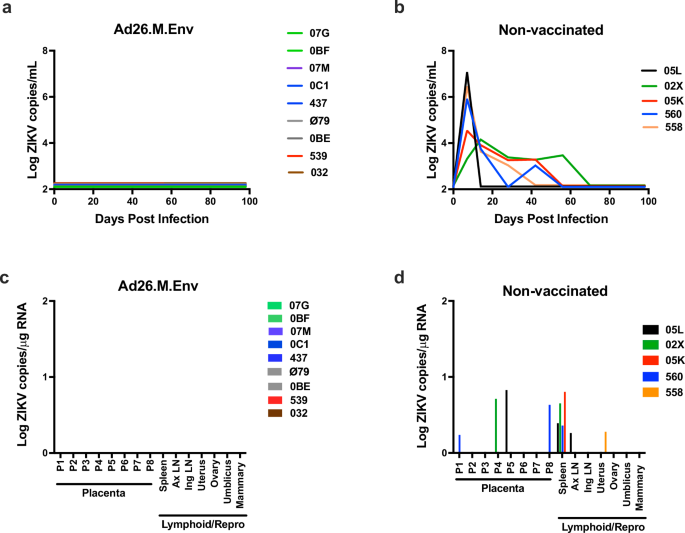
Pregnant Ad26.M.Env vaccinated ( n = 9) and non- vaccinated ( n = 5) macaques were challenged with 1×10 6 vp of ZIKV at 6 weeks following conception and sera was monitored longitudinally for 100 days following challenge. ZIKV log10 copies per mL of sera from ( a ) Ad26.M.Env vaccinated dams and ( b ) non-vaccinated dams were determined by qRT-PCR and depicted as log10 ZIKV copies/mL sera at days 0, 7, 14, 28, 42, 56, 70, 84, and 98. The limit of detection of this assay was 100 copies/mL sera (2Log10). a Individual animals of group 1 (Ad26.M.Env immunized) are color coded to represent number of weeks between immunization and challenge; green (10wk); purple (12 weeks); blue (14 weeks); gray (18 weeks); red (22 weeks); brown (30 weeks). Lymphoid and reproductive tissues were collected from dams at time of Cesarian section (C-section). C-sections were performed 10–14 days prior to estimated full-term delivery dates (~21–23 weeks pregnancy) at approximately 16 weeks following ZIKV challenge. Viral RNA was determined by RT-PCR for ( c ) Ad26.M.Env vaccinated and ( d ) non-vaccinated dams on tissues collected at necropsy. AxLN axillary lymph node, IngLN inguinal LN.
After confirmation of pregnancy, dams were monitored monthly for fetal biometric analyses including measurements of biparietal diameter, occipitofrontal length, head circumference, and femur length by ultrasonography. No abnormalities were noted in fetal biometric parameters in all study groups (Supplementary Fig. 4 ) and fetal brain weights and brain:fetal body weight ratios at necropsy were similar across groups (Supplementary Fig. 5 ).
Dams had scheduled Cesarian sections (C-section) and euthanasia when fetuses were term, approximately 2 weeks prior to estimated delivery date. Maternal tissues previously shown to have detectable virus throughout pregnancy were collected for evaluation by RT-PCR for ZIKV vRNA 33 ). None of the nine Ad26.M.Env vaccinated dams had detectable vRNA in any tissues surveyed (Fig. 3c ). All non-vaccinated, challenged dams had detectable vRNA in at least one of the analyzed tissues. 4/5 animals showed positive vRNA in maternal spleen, consistent with previous reports 23 , 33 , 34 ), and one dam had vRNA detected in the axillary LN. One dam had detectable vRNA in the uterus, and 3/5 dams had virus detectable in the placenta (Fig. 3d ). Placental pathology was evaluated for dams in all groups by both a veterinary pathologist and a human gynecological pathologist specializing in placental histopathology. Histopathological placental findings in all groups were typical of near-term/term placentas in macaques with evidence of maternal thrombosis and infarction in all groups (Supplementary Fig. 5 , Table 1 ) 46 . Fetal: placental ratios were within the expected limits for term fetuses and did not vary significantly between groups (Supplementary Fig. 5 ).
Neonates born to Ad26.M.Env vaccinated dams were negative for ZIKV vRNA and had no ZIKV-associated histopathological abnormalities
Following C-section and euthanasia, fetuses were inspected for gross abnormalities and fetal tissues collected for histopathology and evaluation of vRNA. Fetuses of vaccinated dams had no evidence of viral replication in tissues (Fig. 4a , upper) while 2/5 fetuses from non-vaccinated, challenged dams had detectable virus in tissues, one of which (Fetus 560) had extensive detection of ZIKV in the brain (Fig. 4a , lower). Histopathologic evaluation of brain from fetuses born to Ad26.M.Env vaccinated dams showed no evidence of previously reported ZIKV neuropathology including microcalcifications and perivascular edema (Fig. 4b–e , Fig. 5 ). Fetuses from non-vaccinated, challenged dams had a myriad of abnormal findings (Table 1 ) including a gross cerebellar malformation (Fig. 4f ), asymmetry of the left parietal lobe (Supplementary Fig. 6 , Table 1 ), and a gross dystrophic calcification on the liver (Table 1 ), focal edema (Fig. 4g ), microcalcification (Fig. 4h ), and meningeal proliferation (Fig. 4i ). However, the fetuses from the non-vaccinated, challenged dams overall had fewer histopathological findings than previously reported (Fig. 5 ) 33 . Tissues that were positive for ZIKV by qPCR were evaluated by IHC and RNAscope ISH, but virus was not detected within lesions therefore gross and histological abnormalities in fetuses from non-vaccinated, challenged dams could not be definitively linked to previous or on-going ZIKV viral replication.

Necropsy and collection of fetal tissues were performed on fetuses following euthanasia after term Cesarian delivery. Each dam had only a single fetus. a RT-PCR was performed on fetal tissues in fetuses from Ad26.M.Env vaccinated and ZIKV-challenged dams (upper) and non-vaccinated and ZIKV-challenged dams (lower). Formalin-fixed gross specimens and histopathological changes in fetuses from Ad26.M.Env vaccinated and ZIKV challenged dams ( b – e ) as compared to non-vaccinated and ZIKV challenged dams ( f – i ) showing normal cerebellar folio ( b ; dotted white line), vasculature within cerebellum ( c ), midbrain progenitor cells ( d ), and meninges ( e ) as compared to cerebellar dysplasia ( f , dotted white line), perivascular edema of cerebellar vessel ( g ), microcalcification within midbrain progenitor cells ( h ), and unusual thickening of the meninges ( i ) in macaque infants from non-vaccinated dams. AxLN axillary lymph node, IngLN inguinal LN, Mes LN mesenteric lymph node, PFC prefrontal cortex, FC frontal cortex, BG1 basal ganglia section 1, HC/TH hippocampus, thalamus, OC occipital lobe; CB cerebellum, BS brain stem, CSC cervical spinal cord, TSC thoracic spinal cord, UC umbilical cord. Scale bars = 200 uM ( d , h ); 500 uM ( c , e , g , i ).

Summary of neuropathologic lesions in the CNS (see also Table 1 ). Scoring system is defined as previously described in ref. 33 . PFC prefrontal cortex, FC frontal cortex, BG basal ganglia, TH thalamus, SN substantia nigra, HC hippocampus, OC occipital cortex, PC parietal cortex, TC temporal cortex, CB cerebellum, BS brain stem, CSC cervical spinal cord, TSC thoracic spinal cord, LSC lumbosacral spinal cord, DRG dorsal root ganglia.
ZIKV viral infections world-wide resulted in a pandemic of fetal malformations and fetal loss in pregnant women in 2016. The repercussions of this devasting disease will continue to manifest itself as cohorts of exposed women and children continue to be followed. Although massive ZIKV exposure across endemic regions of South America has likely resulted in elevated neutralizing antibodies in exposed populations, waning of immunity over time will likely lead to cyclical re-emergence of ZIKV as seen with previous outbreaks in Malaysia. The United States is at risk for the re-emergence of a future ZIKV pandemic since the Aedes aegypti mosquito vector is endemic to the southern United States. The lack of exposure of the US population to ZIKV during the 2016 epidemic makes US individuals vulnerable to future ZIKV epidemics. Development of a well-tolerated and safe vaccine that can be used pre- and perinatally is critical to protecting naïve individuals during future ZIKV outbreaks.
The Ad26.M.Env (Ad26.ZIKV.001) vaccine was tested in a Phase I clinical trial and all regimens tested were well tolerated, with no safety concerns identified. Vaccination induced robust ZIKV neutralizing titers that were of similar magnitude to those induced in macaques in this study 22 . In addition, transfer of immune sera from vaccinated study participants to mice protected mice against ZIKV vRNA in blood 22 . Viral vectored vaccines that are immunogenic with a single immunization remain an attractive option for use in resource-poor settings where vaccine access may be limited. The Ad26.CoV2.S vaccine construct was widely deployed during the COVID-19 pandemic and was protective against severe disease and variant infection after a single or prime-boost immunization 47 , 48 . In addition, Ad26.CoV2.S showed durable neutralizing antibody responses which may offer some advantages over similar mRNA constructs in resource-limited settings or during outbreak scenarios 49 . Currently an Ad26 Ebola vaccine (Ad26.ZEBOV) is licensed and recommended as a childhood vaccine in Ebola endemic regions 50 . Although, adenoviral based vaccines used to combat COVID-19 were associated with immune-mediated thrombocytopenia and thrombosis, the mechanisms for these AE have not been fully elucidated, and more work is required to determine if these AE are related to the interplay between the Adenovirus based vaccine and the COVID-19 spike protein, or to the Adenovirus vaccine platform itself and can be avoided by improvements in vaccine platform. mRNA based vaccines against ZIKV have also been shown to be immunogenic and protective in NHP challenge studies, with variable efficacy in inducing neutralizing antibody responses in people 18 , 20 . Nevertheless, adenoviral constructs remain one of the most studied vaccine vectors and will likely continue to be an important construct for global use.
Here we show that an Ad26.M.Env vaccine is safe and efficacious against preventing ZIKV vRNA in sera and tissue of pregnant rhesus macaques. Antibody titers induced by A26.M.Env ZIKV vaccination in macaques were of similar magnitude as those induced by vaccination in a Phase I clinical trial in people 22 and to titers induced by the inactivated Zika virus vaccine (ZPIV) 19 . While current low levels of ZIKV worldwide will impede the ability to conduct prevention of infection studies in people, studies of ZIKV vaccine protection in non-human primates provide critical data that can be extrapolated for immunobridging studies as has been done with Ebola vaccines 51 , 52 . Vaccine induced neutralizing antibodies against ZIKV have been shown to be sufficient for protection in mice that have undergone CD4+ and CD8 + T cell depletions 15 . Similar studies have not been performed to confirm that Ab titers alone are sufficient for maternal protection during pregnancy. Notably, Ad26.M.Env also induced robust anti-Env CD4+ and CD8 + T cell responses. Further work is necessary to determine if cellular immune responses offer improved quality of protection during pregnancy. In both this study and the Phase I clinical trial with Ad26.ZIKV.001 22 ) no anti-PrM cellular immune responses were observed. This may be the consequence of limited antigen size or immunodominance of the Env epitope.
This study had several limitations that prevented us from assessing the full potential of the ZIKV Ad26.M.Env in protecting pregnant dams. In our effort to capture fetal neuropathology, we allowed the pregnancies to continue to term which limited our ability to study ZIKV associated placental pathology which has been well-described in the macaque and marmoset models of ZIKV infection during pregnancy 53 . Similarly, we did not sacrifice fetuses at timepoints where unvaccinated dams remained viremic in plasma, which limited our ability to more rigorously determine if vaccination protects against virus in the placenta and fetal brain during periods of robust systemic viral replication. In addition, fewer neuropathological findings were detected in fetuses from non-vaccinated, challenged dams in the current study as compared to our previous report 33 highlighting the need for studies with large numbers of animals to capture the full spectrum of fetal outcomes. Such large-scale studies are typically not possible due to restrictions on the use of NHP for research and limitations in animal availability. Likewise, we observed two gross anomalies in the fetal brains of non-vaccinated, ZIKV challenged animals, but given the limited evaluation of fetal macaque brains at term in normal colony macaques, we cannot rule out that the gross lesions observed represent normal variation in macaque brains despite their absence in the vaccinated group. Furthermore, questions remain regarding the potential role for previous infection with other related viruses such as Dengue fever virus (DENV) in predisposing to ZIKV-induced development of CZS 54 . Recent studies in both pregnant macaques and marmosets have shown evidence of enhanced neuropathology and placental pathology in previously DENV exposed, ZIKV infected animals 55 , 56 . Future studies on ZIKV vaccine efficacy in DENV pre-exposed dams may be warranted. Lastly, we were unable to follow a cohort of infants longitudinally in this study to determine whether vaccination of dams prevented neurocognitive deficits in offspring 57 ).
All non-vaccinated dams in this study had persistent virus detected in lymphoid organs at term, supporting that ZIKV infection during pregnancy can have severe consequences for pregnant women in the absence of clinical signs. Here we show that vaccination with Ad26.M.Env prevents persistent ZIKV replication in lymphoid organs, placenta and fetal tissue including brain in the non-human primate model with a single immunization. Future studies evaluating Ad26.M.Env protection during the acutely viremic phase in pregnant macaques would extend these findings. These data combined with the Phase I safety and immunogenicity data in people suggests that the Ad26.M.Env is likely to be efficacious in preventing maternal ZIKV infection.
Experimental model and subject details
Outbred, healthy Indian-origin female rhesus monkeys ( Macaca mulatta ) were housed at Alphagenesis, Yemassee, SC. Animals selected for this study were research naive. Upon arrival and standard quarantine procedures, animals were tested for tuberculosis (TB) at least three times at intervals of two weeks. Animals were also screened for Herpes B, Simian retrovirus (SRV), Simian Immunodeficiency Virus (SIV), Simian T-cell Leukemia Virus (STLV), and Measles. All animals were Herpes B, SRV, SIV, and STLV negative. Study animals were selected based on age. Females were all aged 4- 8 years old with similar age and weight distribution per study group. The study protocol was reviewed and approved by the Alphagenesis Institutional Care and Use Committee (IACUC). All experiments conformed to regulatory standards outlined by the American Veterinary Medical Association (AVMA) and American Association of Laboratory Animal Medicine (AALAM).
Breeding, immunization, and ZIKV challenge
Seven weeks prior to immunization, females were removed from their breeding group. For Group 1 (Ad26.M.Env vaccinated, ZIKV-challenge), nine dams that were not pregnant according to ultrasound were intramuscularly immunized with 1×10 11 vp Ad26.M.Env. The nine immunized dams were reintroduced to their breeding groups 17 days later. All animals were provided enrichment according to recommended guidelines. Dams were monitored for pregnancy every 2 weeks by ultrasound until confirmed pregnant. After confirmed pregnancy, dams were monitored by ultrasound every 4 weeks. For Group 1, all nine dams had confirmed pregnancy. For Group 2 (non-vaccinated, ZIKV-challenge controls), 7 dams were included in the study, of which 5 were confirmed pregnant. Pregnant control dams (Group 3, no vaccination, no ZIKV-challenge) were included from the breeding colony and were of similar age and source as vaccinated and non-vaccinated, challenged animals (Groups 1&2).
Approximately six weeks after calculated date of conception (based on ultrasound results), equivalent to human 1st trimester of pregnancy, dams from groups 1 and 2 were challenged with 1 × 10 6 vp (10 3 PFU) of ZIKV-BR via the subcutaneous route. During pregnancy, blood and PBMCs were isolated for immunogenicity readouts. Plasma, cerebrospinal fluid (CFS), urine, colorectal biopsies, inguinal/axillary lymph node (LN) biopsies, rectal, vaginal and saliva secretion were taken to monitor ZIKV vRNA. At approximately week 21–23 of pregnancy ( ≈ week 16 following challenge) fetuses of all groups were delivered by C- section except for one (OBE) from the vaccine group that was born naturally due to earlier than predicted delivery. One pregnancy was lost in the vaccine group due to culture confirmed staphylococcal placentitis (539) and this dam/infant pair was removed from the study. Reproductive failure or preterm delivery is significant among primates, and the observations in this study were within the historical control range for the testing facility (AGI), and within expected outcomes for pregnancies in rhesus monkeys.
Five live male infants and three live female infants were delivered among the vaccinated, challenged group. Three live male infants and two live female infants were delivered to the non-vaccinated, challenged group. Two live males and one live female infant were delivered to the control group (non-vaccinated, non-ZIKV challenged; Table 1 ). Dams and fetuses were euthanized for post-mortem gross pathology, histopathology, and virologic assessments.
Method details
Complete details about the construction and production of the Ad26.M.Env construct are available 15 , 16 , 22 . Briefly, the vaccine was produced on the human PER.C61 cell line and purified and characterized as described previously in ref. 58 . Ad26 particle concentrations were determined by optical density at 260 nm and viral infectivity by TCID50 assay. All vaccine preparations were tested for bioburden and endotoxin levels (MicroSafe, Millipore, Leiden, The Netherlands) and have passed pre-set release criteria for animal experiments.
ZIKV challenge stock preparation
ZIKV-BR (Brazil ZKV2015) was propagated in Vero cells (World Health Organization, NICSC-011038011038) that were maintained in EMEM media supplemented with 10%FBS, 6mM L-glutamine and 1x pen/strep. Cells were passaged twice a week and incubated at 37 °C, 10% CO2.
Ultrasonography
Ultrasounds were performed every 2–4 weeks in the ZIKV-infected pregnant rhesus monkeys as well as in 3 uninfected pregnant rhesus monkeys in the same breeding facility. Animals were sedated with Telazol (5 mg/kg), and a GE Logic E with an 8CRS Micro-convex transducer (FOV 132, 3.6–10 MHz) was used for multiparameter biometric measurements, including biparietal diameter (BPD), occipitofrontal diameter (OFD), head circumference (HC), crown-rump length (CRL), abdominal circumference (AC), and femur length (FL).
Amniocentesis
Animals were sedated with Telazol HCL (4–7 mg/kg IM). The area on the abdomen was clipped and sterilely prepped with triple alternating applications of betadine and alcohol. Using sterile technique, a 22-gauge 3.34-inch needle on a 3-cc syringe was inserted into the ventral abdomen to the amniotic sac with ultrasound guidance. 2 cc of amniotic fluid was collected and frozen immediately.
RT-PCR assays were utilized to monitor viral loads in plasma, CSF, lymph node biopsies, colorectal biopsies, colorectal weck samples, and urine longitudinally every 2–4 weeks as indicated in the experimental design (see Fig. 1a ) and amniotic fluid collected by amniocentesis at day 14 post-ZIKV infection, and from tissues collected at necropsy, essentially as previously described in refs. 15 , 16 , 26 , 33 . RNA was extracted with a QIAcube HT (Qiagen, Germany). Liquid samples were extracted using the Qiacube 96 Cador pathogen HT, and tissue samples were lysed in Qiazol, using the Tissuelyser II (Qiagen, Germany), chloroform treated and extracted with the Qiacube 96 RNeasy HT kit. The wildtype ZIKV BeH815744 Cap gene was utilized as a standard. RNA standards were generated using the AmpliCap-Max T 7 High Yield Message Maker Kit (Cell Script) and purified with RNA clean and concentrator kit (Zymo Research, CA, USA). RNA quality and concentration was assessed by the BIDMC Molecular Core Facility. Log dilutions of the RNA standard were reverse transcribed and included with each RT-PCR assay. Viral loads were calculated as virus particles (VP) per microgram of total RNA as measured on the NanoDrop (Thermo Scientific, Waltham, MA, USA) or as VP per million cells, as shown in Figs. 3 and 4 . Assay sensitivity was >100 copies/mL, >100 copies per million cells, and >3 copies/mg total RNA.
Neutralization Assays
ZIKV-specific neutralizing antibodies were measured by fold reduction neutralization (FRNT) and microneutralization (MN) assays, as previously described in refs. 15 , 16 , 26 , 33 . For FRNT assay Vero cells were seeded at a concentration of 2 × 10 4 cells/well in 96-well plates 24 h prior to the assay initiation. Heat inactivated serum samples were serially diluted prior to being mixed and incubated with input virus ZIKV-PR (PRVABC59) for 1 hour at 37 °C. Cell-seeded 96-well plates were infected with 100 μL of the virus/serum mixtures for 1 hour before the addition of overlay media. Each serum dilution was tested in triplicate wells. Approximately 24 h after infection, ZIKV foci were detected using an antiflavivirus detection antibody, a horseradish peroxidase (HRP)-conjugated secondary antibody and True-Blue peroxidase substrate. ZIKV foci were visualized and counted using an ImmunoSpot analyzer and software. Each assay run included virus input and media-only control wells, as well as negative and positive control serum samples. Neutralizing antibody titers were reported as the inverse of the serum dilution estimated to reduce the number of input virus by 50% (FRNT50) as shown in Fig. 1 .
Microneutralization assays were performed at WRAIR. Serum samples were serially diluted three-fold in 96-well micro-plates, in a total volume of 100 uL. 10 2 PFU ZIKV-PR (PRVABC59) in a total volume of 100uL was added and incubated at 35 °C for 2 h. Serum/virus mixtures were then transferred to microtiter plates containing confluent Vero cell monolayers (World Health Organization, NICSC-011038011038). After incubation for 4 days, cells were fixed with absolute ethanol: methanol for 1 h at –20 °C and washed three times with PBS. The pan-flavivirus monoclonal antibody 6B6-C1 conjugated to HRP (6B6-C1 was a gift from JT Roehrig, CDC) was then added to each well, incubated at 35 °C for 2 h, and washed with PBS. Plates were washed, developed with 3,3’,5,5’–tetramethylbenzidine (TMB) for 50 min at room temperature, stopped with 1:25 phosphoric acid, and absorbance was read at 450 nm. For a valid assay, the average absorbance at 450 nm of three non-infected control wells had to be % 0.5, and virus-only control wells had to be R 0.9. Normalized absorbance values were calculated, the MN50 titer was determined by a log mid-point linear regression model. The MN50 titer was calculated as the reciprocal of the serum dilution that neutralized R 50% of ZIKV, and seropositivity was defined as a titer R10, with the maximum measurable titer 7290, as shown in Supplementary Fig. 2 .
Tissue Collection and Histopathology
Within 14 days of estimated term gestation (26 weeks), dams and fetuses were euthanized with intravenous sodium pentobarbital, and delivery was by caesarian section. Complete necropsies were performed by a veterinarian (A.J.M) on fetuses immediately following euthanasia, utilizing standard necropsy procedures with standard sterile surgical grade necropsy instruments and dissection blades. Briefly, peripheral lymphoid tissues were collected, followed by the gastrointestinal tract and abdominal organs. The pleural cavity was opened and the tongue, pharynx, trachea, esophagus, heart, and lungs (“pluck”) were removed en masse . Reproductive organs were collected, followed by brain, spinal cord, and eyes. Ruskin-Liston bone cutting forceps were used to expose the spinal cord to the level of the cauda equina . Limited necropsies were performed on dams for tissues previously shown to harbor viral RNA including reproductive organs, lymphoid tissues, spleen, and placenta. Fresh tissues were collected utilizing sterile blades for viral RT-PCR in RNAlater (Ambion). Frozen tissue for histopathology was prepared by trimming tissue, placing tissue samples into cryomolds with optimal cutting temperature medium (OCT, Tissue-Tek), and flash freezing on-site. Additional tissues were fixed in 10% neutral buffered formalin (NBF) for histopathology. Formalin-fixed tissues were trimmed, processed, and embedded in paraffin, sectioned, and stained with hematoxylin and eosin, and evaluated independently by two blinded veterinary pathologists (A.J.M., R.B.). Placenta was evalualated by a blinded gynecologic pathologist (J.L.H).
Immunohistochemistry and in situ hybridization
Immunohistochemistry and in situ hybridization (RNAscope TM ) were performed as previously described in ref. 33 . Briefly, tissue sections were deparaffinized in xylene and rehydrated through graded ethanol solutions to distilled water. Endogenous peroxidase activity was blocked by incubation with 3% hydrogen peroxide followed by heat induced epitope retrieval (HIER) in citrate buffer (Vector Labs) using a slide steamer (IHC World). Tissues were treated for nonspecific protein binding (Protein Block, DAKO) followed by application of mouse-anti ZIKV envelope (BioFront Technologies; BF-1176-56, 1:200) for 30 min at room temperature. A biotin-free polymer-based alkaline phosphatase kit with Permanent Red was used to detect antigen-antibody complexes (Polink-1 AP, Golden Bridge International Labs; #D18-18). In situ detection of ZIKV RNA was performed using RNAscope (ACDBio) technology. The ZIKV Asian probe (formerly O4, #468361) and red detection kit were used according to the manufacturer’s instructions.
ZIKV-specific cellular immune responses were assessed by IFN-γ ELISPOT assays shown in Fig. 2 using pools of over- lapping 15-amino-acid peptides covering the prM and Env proteins (JPT, Berlin, Germany), essentially as we previously described in ref. 16 . 96-well multiscreen plates (Millipore, MA, USA) were coated overnight with 100 mL/well of 5 mg/ml anti-human interferon-g (BD Biosciences, CA, USA; BD #554699) in endotoxin-free Dulbecco’s PBS (D-PBS). The plates were then washed three times with D-PBS containing 0.25% Tween 20 (D-PBS-Tween), blocked for 1–4 h with D-PBS containing 5% FBS at 37 °C, and incubated with 2 mg/ml of each peptide and 2 × 10 5 monkey PBMC in triplicate in 100 mL reaction mixture volumes. Following an 18–24 h incubation at 37 °C, the plates were washed nine times with PBS-Tween and incubated for 3 min with distilled water. The plates were then incubated with 1 mg/ml biotinylated anti-human interferon-g (U-Cytech Biosciences, UT, NETH) for 2 h at room temperature, washed six times with PBS-Tween, and incubated for 2 h with streptavidin-alkaline phosphatase (Southern Biotechnology Associates, AL, USA). Following five washes with PBS-Tween and one with PBS, the plates were developed with nitroblue tetrazolium-5- bromo-4-chloro-3-indolyl-phosphate chromogen (Pierce, IL, USA), stopped by washing with tap water, air-dried, and read using an ELISPOT reader (Cellular Technology Ltd., OH, USA). The numbers of spot-forming cells (SFU) per 10 6 cells were calculated. The medium background levels were typically < 15 SFU per 10 6 cells. SFU per 10 6 PBMCs of unstimulated PBMCs was subtracted from specific responses of corresponding individual macaques. Specific responses that were at or below zero after background subtraction were set to 1.
Monkey ZIKV NS1 ELISA kits (Alpha Diagnostic International, TX, USA) were used to determine endpoint binding antibody titers using a modified protocol 16 . 96-well plates coated with ZIKV NS1 protein (RV-403310-1 Alpha Diagnostics) were first equilibrated at room temperature with 300 ml of kit working wash buffer for 5 min. 6 ml of monkey serum was added to the top row, and 3-fold serial dilutions were tested in the remaining rows. Serum samples were incubated at room temperature for 1 hr, and plates washed 4 times. 100 mL of anti-monkey IgG HRP-conjugate working solution was then added to each well and incubated for 30 min at room temperature. Plates were washed 5 times, developed for 15 min at room temperature with 100 ml of TMB substrate, and stopped by the addition of 100 ml of stop solution. Plates were analyzed at 450 nm/550 nm on a VersaMax microplate reader using Softmax Pro 6.0 software (Molecular Devices, CA, USA). ELISA endpoint titers were defined as the highest reciprocal serum dilution that yielded an absorbance > 2-fold over background values and plotted as Log 10 endpoint titer.
Data availability
All data generated and analyzed in this study are available from the Lead Contact upon reasonable request.
Brasil, P. et al. Zika virus infection in pregnant women in Rio de Janeiro. N. Engl. J. Med. 375 , 2321–2334 (2016).
Article PubMed PubMed Central Google Scholar
Driggers, R. W. et al. Zika virus infection with prolonged maternal viremia and fetal brain abnormalities. N. Engl. J. Med. 374 , 2142–2151 (2016).
Article CAS PubMed Google Scholar
Hoen, B. et al. Pregnancy outcomes after ZIKV infection in french territories in the Americas. N. Engl. J. Med. 378 , 985–994 (2018).
Article PubMed Google Scholar
Haby, M. M., Pinart, M., Elias, V. & Reveiz, L. Prevalence of asymptomatic Zika virus infection: a systematic review. Bull. World Health Organ. 96 , 402–413D (2018).
Langerak, T. et al. Transplacental Zika virus transmission in ex vivo perfused human placentas. Plos Negl. Trop. D. 16 , e0010359 (2022).
Article CAS Google Scholar
Adibi, J. J., Marques, E. T. A. Jr, Cartus, A. & Beigi, R. H. Teratogenic effects of the Zika virus and the role of the placenta. Lancet 387 , 1587–1590 (2016).
Vasquez, A. M., Sapiano, M. R. P., Basavaraju, S. V., Kuehnert, M. J. & Rivera-Garcia, B. Survey of blood collection centers and implementation of guidance for prevention of transfusion-transmitted Zika virus. Infect. — Puerto Rico, 2016. Mmwr Morbidity Mortal. Wkly Rep. 65 , 375–378 (2016).
Google Scholar
Oster, A. M. et al. Update: interim guidance for prevention of sexual transmission of Zika virus — United States, 2016. Morbidity Mortal. Wkly Rep. 65 , 323–325 (2016).
Article Google Scholar
Santos, C. N. O. et al. Association between genetic variants in TREM1, CXCL10, IL4, CXCL8 and TLR7 genes with the occurrence of congenital Zika syndrome and severe microcephaly. Sci. Rep. 13 , 3466 (2023).
Article CAS PubMed PubMed Central Google Scholar
WHO. Situation Report: Zika Virus, Microcephaly and Guillain Barre Syndrome 3 November, 2016 . (WHO< 2016).
Vannice, K. S. et al. Demonstrating vaccine effectiveness during a waning epidemic: A WHO/NIH meeting report on approaches to development and licensure of Zika vaccine candidates. Vaccine 37 , 863–868 (2019).
Christie, C. D. C., Lue, A. M. & Melbourne-Chambers, R. H. Dengue, chikungunya and zika arbovirus infections in Caribbean children. Curr. Opin. Pediatr. 35 , 155–165 (2023).
CDC. CDC Traveler’s Health:Zika . https://wwwnc.cdc.gov/travel/diseases/zika .
Lunardelli, V. A. S., Apostolico, J. D. S., Fernandes, E. R. & Rosa, D. S. Zika virus—an update on the current efforts for vaccine development. Hum. Vacc. Immunother. 17 , 1–5 (2020).
Larocca, R. A. et al. Vaccine protection against Zika virus from Brazil. Nature 536 , 474–478 (2016).
Abbink, P. et al. Protective efficacy of multiple vaccine platforms against Zika virus challenge in rhesus monkeys. SCIENCE 353 , 1129–1132 (2016).
Richner, J. M. et al. Modified mRNA vaccines protect against Zika virus infection. Cell 168 , 1114–1125.e10 (2017).
Bollman, B. et al. An optimized messenger RNA vaccine candidate protects non-human primates from Zika virus infection. npj Vaccines 8 , 58 (2023).
Stephenson, K. E. et al. Safety and immunogenicity of a Zika purified inactivated virus vaccine given via standard, accelerated, or shortened schedules: a single-centre, double-blind, sequential-group, randomised, placebo-controlled, phase 1 trial. Lancet Infect. Dis. 20 , 1061–1070 (2020).
Essink, B. et al. The safety and immunogenicity of two Zika virus mRNA vaccine candidates in healthy flavivirus baseline seropositive and seronegative adults: the results of two randomised, placebo-controlled, dose-ranging, phase 1 clinical trials. Lancet Infect. Dis. 23 , 621–633 (2023).
Modjarrad, K. et al. Preliminary aggregate safety and immunogenicity results from three trials of a purified inactivated Zika virus vaccine candidate: phase 1, randomised, double-blind, placebo-controlled clinical trials. Lancet 391 , 563–571 (2018).
Salisch, N. C. et al. A double-blind, randomized, placebo-controlled phase 1 study of Ad26.ZIKV.001, an Ad26-vectored anti-Zika virus vaccine. Ann. Intern. Med. 174 , 585–594 (2021).
Dudley, D. M. et al. A rhesus macaque model of Asian-lineage Zika virus infection. Nat. Commun. 7 , 12204 (2016).
Coffey, L. L. et al. Zika virus tissue and blood compartmentalization in acute infection of rhesus macaques. PLoS ONE 12 , e0171148–15 (2017).
Osuna, C. E. et al. Zika viral dynamics and shedding in rhesus and cynomolgus macaques. Nat. Med. 22 , 1448–1455 (2016).
Aid, M. et al. Zika virus persistence in the central nervous system and lymph nodes of rhesus monkeys. Cell 169 , 610–620.e14 (2017).
Magnani, D. M. et al. Fetal demise and failed antibody therapy during Zika virus infection of pregnant macaques. Nat. Commun. 9, 1624 (2018) https://doi.org/10.1038/s41467-018-04056-4 .
Raasch, L. E. et al. Fetal loss in pregnant rhesus macaques infected with high-dose African-lineage Zika virus. Plos Negl. Trop. D. 16 , e0010623 (2022).
Ausderau, K. et al. Neonatal development in prenatally Zika virus-exposed infant macaques with dengue immunity. Viruses 13 , 1878 (2021).
Mohr, E. L. et al. Ocular and uteroplacental pathology in a macaque pregnancy with congenital Zika virus infection. PLoS ONE 13 , e0190617–e0190628 (2018).
Crooks, C. M. et al. Previous exposure to dengue virus is associated with increased Zika virus burden at the maternal-fetal interface in rhesus macaques. Plos Negl. Trop. D. 15 , e0009641 (2021).
Hirsch, A. J. et al. Zika virus infection in pregnant rhesus macaques causes placental dysfunction and immunopathology. Nat. Commun. 9 , 263 (2018).
Martinot, A. J. et al. Fetal neuropathology in Zika virus-infected pregnant female rhesus monkeys. Cell 173 , 1111–1122.e10 (2018).
Dudley, D. M. et al. Miscarriage and stillbirth following maternal Zika virus infection in nonhuman primates. Nat. Med. 24 , 1104–1107 (2018).
Waldorf, K. M. A. et al. Congenital Zika virus infection as a silent pathology with loss of neurogenic output in the fetal brain. Nat. Med. 24 , 368–374 (2018).
Nguyen, S. M. et al. Highly efficient maternal-fetal Zika virus transmission in pregnant rhesus macaques. PLoS Pathog. 13 , e1006378–22 (2017).
Rompay, K. K. A. V. et al. DNA vaccination before conception protects Zika virus-exposed pregnant macaques against prolonged viremia and improves fetal outcomes. Sci. Transl. Med. 11 , eaay2736 (2019).
Gebre, M. S. et al. Novel approaches for vaccine development. Cell 184 , 1589–1603 (2021).
Barouch, D. H. et al. International seroepidemiology of adenovirus serotypes 5, 26, 35, and 48 in pediatric and adult populations. Vaccine 29 , 5203–5209 (2011).
Barouch, D. H., Thomas, S. J. & Michael, N. L. Prospects for a Zika Virus Vaccine. Immunity 46 , 176–182 (2017).
Cox, F. et al. Adenoviral vector type 26 encoding Zika virus (ZIKV) M-Env antigen induces humoral and cellular immune responses and protects mice and nonhuman primates against ZIKV challenge. PLoS ONE 13 , e0202820–19 (2018).
Larocca, R. A. et al. Adenovirus vector-based vaccines confer maternal-fetal protection against Zika virus challenge in pregnant IFN-αβR−/− mice. Cell Host Microbe 26 , 591–600.e4 (2019).
Miner, J. J. et al. Zika virus infection during pregnancy in mice causes placental damage and fetal demise. Cell 165 , 1081–1091 (2016).
Waldorf, K., Stencel-Baerenwald, J. E. & Kapur, R. P. Fetal brain lesions after subcutaneous inoculation of Zika virus in a pregnant nonhuman primate. Nat. Med. 22 , 1256–1259 (2016).
Coffey, L. L. et al. Intraamniotic Zika virus inoculation of pregnant rhesus macaques produces fetal neurologic disease. Nat. Commun. 9 , 2414 (2018).
Cline, J. M. et al. The placenta in toxicology. Part III: Pathologic assessment of the placenta. Toxicol. Pathol. 42 , 339–344 (2014).
Sadoff, J. et al. Safety and efficacy of single-dose Ad26.COV2.S vaccine against Covid-19. N. Engl. J. Med. 384 , 2187–2201 (2021).
Hardt, K. et al. Efficacy, safety, and immunogenicity of a booster regimen of Ad26.COV2.S vaccine against COVID-19 (ENSEMBLE2): results of a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Infect. Dis. 22 , 1703–1715 (2022).
Prather, A. A. et al. Predictors of long-term neutralizing antibody titers following COVID-19 vaccination by three vaccine types: the BOOST study. Sci. Rep. 13 , 6505 (2023).
Manno, D. et al. Safety and immunogenicity of an Ad26.ZEBOV booster dose in children previously vaccinated with the two-dose heterologous Ad26.ZEBOV and MVA-BN-Filo Ebola vaccine regimen: an open-label, non-randomised, phase 2 trial. Lancet Infect. Dis. 23 , 352–360 (2023).
Bockstal, V. et al. Non-human primate to human immunobridging demonstrates a protective effect of Ad26.ZEBOV, MVA-BN-Filo vaccine against Ebola. npj Vaccines 7 , 156 (2022).
Roozendaal, R. et al. Nonhuman primate to human immunobridging to infer the protective effect of an Ebola virus vaccine candidate. npj Vaccines 5 , 112 (2020).
Kim, I.-J. et al. Protective efficacy of a Zika purified inactivated virus vaccine candidate during pregnancy in marmosets. npj Vaccines 9 , 35 (2024).
Carvalho, M. S., Freitas, L. P., Cruz, O. G., Brasil, P. & Bastos, L. S. Association of past dengue fever epidemics with the risk of Zika microcephaly at the population level in Brazil. Sci. Rep. 10 , 1752 (2020).
Saron, W. A. A. et al. Exacerbated Zika virus–induced neuropathology and microcephaly in fetuses of dengue-immune nonhuman primates. Sci. Transl. Med. 15 , eadd2420 (2023).
Kim, I.-J. et al. Impact of prior dengue virus infection on Zika virus infection during pregnancy in marmosets. Sci. Transl. Med. 15 , eabq6517 (2023).
Raper, J. et al. Long-term alterations in brain and behavior after postnatal Zika virus infection in infant macaques. Nat. Commun. 11 , 2534 (2020).
Abbink, P. et al. Comparative seroprevalence and immunogenicity of six rare serotype recombinant adenovirus vaccine vectors from subgroups B and D. J. Virol. 81 , 4654–4663 (2007).
Download references
Acknowledgements
We acknowledge Abishek Chandrashekar and Brianna Altimonti for scientific discussions and technical support. We acknowledge funding from Janssen, the Ragon Institute of MGH, MIT, and Harvard, and NIH NIAID K08 135098-01A1.
Author information
Authors and affiliations.
Center for Virology and Vaccine Research, Beth Israel Deaconess Medical Center, Harvard Medical School, Boston, MA, USA
Amanda J. Martinot, Peter Abbink, Erica N. Borducchi & Dan H. Barouch
Departments of Infectious Disease and Global Health and Comparative Pathobiology, Cummings School of Veterinary Medicine, Tufts University, North Grafton, MA, USA
Amanda J. Martinot
Janssen Vaccines & Prevention, Leiden, the Netherlands
Freek Cox, Roland Zahn & Leslie van der Fits
Division of Anatomic Pathology, Beth Israel Deaconess Medical Center, Boston, MA, USA
Jonathan L. Hecht
Harvard Medical School, Boston, MA, USA
Roderick Bronson
Alphagenesis, Yemassee, SC Alphagenesis, Yemassee, SC, USA
William J. Rinaldi & Melissa J. Ferguson
Walter Reed Army Institute of Research, Silver Spring, MD, USA
Rafael A. De La Barrera
Ragon Institute of MGH, MIT and Harvard, Cambridge, MA, USA
Dan H. Barouch
You can also search for this author in PubMed Google Scholar
Contributions
DHB, RZ, LF, FC conceptualized and designed the study; WJR, MJF, AJM, AC, and FC supervised and conducted the NHP studies; AJM, RB, and JLH performed the pathological analyses. PA supervised all virologic assays. RAB performed the neutralization assays. FC, ENB, LF, and AJM curated and analyzed the data; AJM wrote the paper; FC, LF, RZ, and DHB reviewed and edited the manuscript.
Corresponding authors
Correspondence to Amanda J. Martinot or Dan H. Barouch .
Ethics declarations
Competing interests.
LF, RZ, and FC are or were employees of Janssen Vaccines & Prevention B.V. DHB and PA are co-inventors on the patent PCT/US2017/036900 (Compositions and methods for preventing and treating ZIKA virus infection). All other authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary information, rights and permissions.
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/ .
Reprints and permissions
About this article
Cite this article.
Martinot, A.J., Cox, F., Abbink, P. et al. Ad26.M.Env ZIKV vaccine protects pregnant rhesus macaques and fetuses against Zika virus infection. npj Vaccines 9 , 157 (2024). https://doi.org/10.1038/s41541-024-00927-8
Download citation
Received : 15 February 2024
Accepted : 23 July 2024
Published : 28 August 2024
DOI : https://doi.org/10.1038/s41541-024-00927-8
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Sign up for the Nature Briefing: Microbiology newsletter — what matters in microbiology research, free to your inbox weekly.
- International
- Today’s Paper
- Javed & Zoya LIVE
- 🇮🇳 I-Day SALE
- Express Shorts
- Mini Crossword
- Health & Wellness
As cases of ‘sloth fever’ continue to rise across Europe and the US, we understand why it poses a significant threat to public health
One of the significant factors contributing to its rapid spread in europe and the us is increased travel and global interconnectedness.
A mysterious and deadly illness known as ‘sloth fever’ is spreading across Europe and the United States, raising concerns among health officials and the public alike. The disease currently has no known cure, making it a significant threat .
In a latest development, more than 20 US travellers returning from Cuba have tested positive, according to an update by the Centers for Disease Control and Prevention on Tuesday. Reports also state that 19 cases have been reported in Europe so far. As cases continue to rise, understanding the nature of this fever, its transmission, and preventive measures is crucial for public health safety.

Primary symptoms
Dr Palleti Siva Karthik Reddy, MBBS, MD, general physician, tells indianexpress.com , “Sloth fever, formally known as Oropouche fever, presents with symptoms that typically resemble other viral infections such as dengue fever . It is caused by Oropouche virus. The primary symptoms include fever, headache, muscle aches, stiff joints, nausea, vomiting, chills, and sensitivity to light.”
Dr Pranav Honnavara Srinivasan MBBS, MD, DM, consultant gastroenterologist at Fortis, adds, “In rare but severe cases, the infection can progress to complications like meningitis or encephalitis, posing a serious risk to the nervous system. The incubation period, or the time between infection and the onset of symptoms, usually ranges from 3 to 8 days.”
How is sloth fever transmitted?
According to Dr Reddy, sloth fever is primarily transmitted through bites from mosquitoes and midges that have fed on infected animals, such as sloths, monkeys, or birds. “This mode of transmission makes it similar to other arboviruses like dengue and Zika .”

One of the significant factors contributing to its rapid spread in Europe and the US is increased travel and global interconnectedness, he states, which facilitates the movement of infected individuals and vectors across borders.
Dr Srinivasan echoes this. He says, “The rapid spread of the virus across Europe and the US is likely attributed to a combination of factors, including increased international travel, climate change expanding the habitats of disease-carrying insects, and a lack of widespread immunity to this relatively new virus.”
What measures can individuals take to reduce their risk of infection?
Since there is no cure or vaccine available for sloth fever, prevention is key, says Dr Reddy. Individuals can reduce their risk of infection by taking the following measures:
– Avoid Insect Bites: Use insect repellents that contain DEET, wear long sleeves and pants, and use mosquito nets, especially when sleeping.
– Minimise Exposure to Vectors: Avoid areas with high mosquito and midge activity, particularly during peak biting times such as dawn and dusk.
– Travel Precautions: For those travelling to areas where the virus is known to be present, staying in accommodations with proper screening or air conditioning can reduce exposure to insects.
– Public Health Awareness: Being aware of travel advisories and health alerts from organisations like the CDC can help travellers take appropriate precautions.
Current challenges faced by the medical community in diagnosing and treating sloth fever
“The diagnosis of sloth fever can be challenging due to its overlapping symptoms with other viral illnesses like dengue or Zika . Currently, there is no specific treatment for the virus , and care focuses on managing symptoms and providing supportive care,” says Dr Srinivasan.
The medical community is actively researching the virus to understand its pathogenesis and develop effective diagnostic tools and antiviral therapies, he mentions. However, the road to a cure or vaccine is likely to be long and complex, emphasising the importance of prevention in the meantime.
DISCLAIMER : This article is based on information from the public domain and/or the experts we spoke to. Always consult your health practitioner before starting any routine.
📣 For more lifestyle news, click here to join our WhatsApp Channel and also follow us on Instagram

Lou Vincent, a former cricketer banned for match-fixing, was honored by Sir Richard Hadlee for his achievements in 100 ODIs. Despite battling depression and addiction, he has found solace in a new life in rural New Zealand with his fiancée. Recently, he made a comeback to cricket by playing for his childhood club in a charity match for men's mental health.

More Lifestyle

Buzzing Now

Aug 29: Latest News
- 01 Maha-Metro prepares draft MoU for Shivajinagar bus station
- 02 Major 911 outage hits US, several cities in California, Texas, Alabama, Ohio affected
- 03 FBI says gunman spent months seeking a target, then settled on Trump
- 04 Woman’s torso found: Cops deploy drones, divers for murder investigation
- 05 India at Paris 2024 Paralympics: Who are India’s top medal contenders? An analysis of 7 podium prospects
- Elections 2024
- Political Pulse
- Entertainment
- Movie Review
- Newsletters
- Web Stories
What to know about the Oropouche virus, also known as sloth fever
More than 20 people returning to the U.S. from Cuba have been infected with a virus transmitted by bugs in recent months, federal health officials said Tuesday. They all had Oropouche virus disease, also known as sloth fever.
By MIKE STOBBE
NEW YORK — More than 20 people returning to the U.S. from Cuba have been infected with a virus transmitted by bugs in recent months, federal health officials said Tuesday. They all had Oropouche virus disease, also known as sloth fever.
None have died, and there is no evidence that it's spreading in the United States. But officials are warning U.S. doctors to be on the lookout for the infection in travelers coming from Cuba and South America.
Here's a look at the illness and what sparked the alert:
What is Oropouche virus?
Oropouche is a virus that is native to forested tropical areas. It was first identified in 1955 in a 24-year-old forest worker on the island of Trinidad, and was named for a nearby village and wetlands.
It has sometimes been called sloth fever because scientists first investigating the virus found it in a three-toed sloth, and believed sloths were important in its spread between insects and animals.
How does Oropouche virus spread?
The virus is spread to humans by small biting flies called midges, and by some types of mosquitoes. Humans have become infected while visiting forested areas and are believed to be responsible for helping the virus make its way to towns and cities, but person-to-person transmission hasn't been documented.
How many cases have there been?
Beginning late last year, the virus was identified as the cause of large outbreaks in Amazon regions where it was known to exist, as well as in new areas in South America and the Caribbean. About 8,000 locally acquired cases have been reported in Bolivia, Brazil, Colombia, Cuba, and Peru.
Some travelers have been diagnosed with it in the U.S. and Europe. The U.S. Centers for Disease Control and Prevention on Tuesday said 21 U.S. cases have been reported so far — 20 in Florida and one in New York — all of whom had been in Cuba. European health officials previously said they had found 19 cases, nearly all among travelers.
What are the symptoms and treatments?
Symptoms can seem similar to other tropical diseases like dengue, Zika or malaria. Fever, headaches and muscle aches are common, and some infected people also suffer diarrhea, nausea, vomiting or rash.
Some patients suffer recurring symptoms, and 1 in 20 can suffer more severe symptoms like bleeding, meningitis and encephalitis. It is rarely fatal, though there are recent reports of deaths in two healthy young people in Brazil.
There are no vaccines to prevent infections and no medicines available to treat the symptoms.
Are there other concerns?
In Brazil, officials are investigating reports that infections might be passed on from a pregnant woman to a fetus — a potentially frightening echo of what was seen during Zika outbreaks nearly a decade ago.
The CDC has recommended that pregnant women avoid non-essential travel to Cuba and suggested all travelers take steps to prevent bug bites, such as using insect repellents and wearing long-sleeved shirts and long pants.
The Associated Press Health and Science Department receives support from the Howard Hughes Medical Institute's Science and Educational Media Group. The AP is solely responsible for all content.
about the writer
Mike stobbe, more from nation, fall is bringing fantasy (and romantasy), literary fiction, politics and taylor-ed book offerings.
Brandon Sanderson, whose epic ''Wind and Truth'' is a highlight of the upcoming publishing season, sees nothing wrong with the idea of ''escapism.''
Israel-Hamas war latest: Israeli military says it killed 5 more militants in West Bank operation
The Israeli military says it has killed five more militants in a large-scale operation in the occupied West Bank, including a well-known local commander.
Authorities: Police gunfire did not kill 8-year-old who was found in the vehicle on an I-95 bridge
A man being pursued in the killing of a woman in New Hampshire was shot by police and tumbled from the Interstate 95 bridge that connects the state to Maine, officials said Thursday. An 8-year-old boy was found dead in his vehicle.
Official websites use .gov
A .gov website belongs to an official government organization in the United States.
Secure .gov websites use HTTPS
A lock ( ) or https:// means you've safely connected to the .gov website. Share sensitive information only on official, secure websites.
Oropouche Virus Disease Among U.S. Travelers — United States, 2024
Early Release / August 27, 2024 / 73
Andrea Morrison, PhD 1 ; Jennifer L. White, MPH 2 ; Holly R. Hughes, PhD 3 ; Sarah J. Guagliardo, PhD 3 ; Jason O. Velez 3 ; Kelly A. Fitzpatrick, MSPH 3 ; Emily H. Davis, PhD 3 ; Danielle Stanek, DVM 1 ; Edgar Kopp, MS 4 ; Peter Dumoulin, PhD 4 ; Timothy Locksmith, MS 4 ; Lea Heberlein, DrPH 4 ; Rebecca Zimler, PhD 1 ; Joshua Lassen, MPH 1 ; Carolina Bestard, MPH 5 ; Edhelene Rico, MPH 5 ; Alvaro Mejia-Echeverri, MD 5 ; Kay-Anna Edwards-Taylor 6 ; Douglas Holt, MD 6 ; Dionisia Halphen, MPH 7 ; Kaitlynn Peters, MHS 8 ; Cheryl Adams 9 ; Amanda M. Nichols, MPH 10 ; Alexander T. Ciota, PhD 11 ; Alan P. Dupuis II 11 ; P. Bryon Backenson, MS 2 ; Jennifer A. Lehman 3 ; Shelby Lyons, MPH 3 ; Hannah Padda, DVM 3 ,12 ; Roxanne C. Connelly, PhD 3 ; Van T. Tong, MPH 13 ; Stacey W. Martin, MSc 3 ; Amy J. Lambert, PhD 3 ; Aaron C. Brault, PhD 3 ; Carina Blackmore, DVM 14 ; J. Erin Staples, MD, PhD 3 ; Carolyn V. Gould, MD 3 ( View author affiliations )
What is already known about this topic?
Oropouche virus is an emerging arthropod-borne virus in the Americas. Recent reports of outbreaks in areas without previous endemic transmission, fatal cases, and vertical transmission associated with adverse pregnancy outcomes have raised concerns about human health risks.
What is added by this report?
As of August 16, 2024, a total of 21 Oropouche virus disease cases among U.S. travelers returning from Cuba have been reported. Most patients had self-limited illness. At least three patients experienced recurrent symptoms after resolution of the initial illness.
What are the implications for public health practice?
Clinicians and public health jurisdictions should be aware of the occurrence of Oropouche virus disease in U.S. travelers and request testing for suspected cases. Travelers should prevent insect bites when traveling, and pregnant persons should consider deferring travel to areas experiencing outbreaks of Oropouche virus disease.
Beginning in late 2023, Oropouche virus was identified as the cause of large outbreaks in Amazon regions with known endemic transmission and in new areas in South America and the Caribbean. The virus is spread to humans by infected biting midges and some mosquito species. Although infection typically causes a self-limited febrile illness, reports of two deaths in patients with Oropouche virus infection and vertical transmission associated with adverse pregnancy outcomes have raised concerns about the threat of this virus to human health. In addition to approximately 8,000 locally acquired cases in the Americas, travel-associated Oropouche virus disease cases have recently been identified in European travelers returning from Cuba and Brazil. As of August 16, 2024, a total of 21 Oropouche virus disease cases were identified among U.S. travelers returning from Cuba. Most patients initially experienced fever, myalgia, and headache, often with other symptoms including arthralgia, diarrhea, nausea or vomiting, and rash. At least three patients had recurrent symptoms after the initial illness, a common characteristic of Oropouche virus disease. Clinicians and public health jurisdictions should be aware of the occurrence of Oropouche virus disease in U.S. travelers and request testing for suspected cases. Travelers should prevent insect bites when traveling, and pregnant persons should consider deferring travel to areas experiencing outbreaks of Oropouche virus disease.
Investigation and Results
Natural history and clinical symptoms.
Oropouche virus (Simbu serogroup, genus Orthobunyavirus ) is endemic to the Amazon region and was previously identified as a cause of human disease in several countries in South and Central America and the Caribbean ( 1 ). The virus circulates in a sylvatic cycle, possibly involving certain vertebrate hosts (e.g., sloths, nonhuman primates, and birds) and mosquitoes, and an urban cycle in which humans serve as amplifying hosts with known vectors being biting midges ( Culicoides paraensis ) and possibly mosquitoes (e.g., Culex quinquefasciatus ) ( 1 ).
The clinical signs and symptoms of Oropouche virus disease are similar to those of other arboviral diseases such as dengue, Zika, and chikungunya. After an incubation period of 3–10 days, patients typically experience abrupt onset of fever, chills, headache, myalgia, and arthralgia. Other symptoms might include retroorbital pain, photophobia, vomiting, diarrhea, fatigue, maculopapular rash, conjunctival injection, and abdominal pain. Initial symptoms usually last only a few days, but up to 70% of patients are reported to have recurrent symptoms within days to weeks after resolution of their initial illness ( 2 ). Although illness is typically mild, hemorrhagic manifestations (e.g., epistaxis, gingival bleeding, melena, menorrhagia, and petechiae) or neuroinvasive disease (e.g., meningitis and meningoencephalitis) can rarely occur ( 1 , 3 , 4 ). No vaccines to prevent or medicines to treat Oropouche virus disease exist; treatment is supportive.
Recent Outbreaks in South America and Cuba
During December 2023–June 2024, large Oropouche virus disease outbreaks were recognized in areas with known endemic disease, and the virus emerged in new areas in South America and Cuba where it had not been historically reported ( 3 ). As of August 2024, over 8,000 laboratory-confirmed cases have been reported in Bolivia, Brazil, Colombia, Cuba, and Peru ( 3 ). These large outbreaks have resulted in travel-associated cases, with 19 Oropouche virus disease cases in European travelers returning from Cuba (n = 18) and Brazil (one) during June–July 2024 ( 5 ). Recently, cases of severe disease leading to two deaths and vertical transmission associated with fetal death and possible congenital malformations in Brazil have raised concerns about the threat of Oropouche virus to human health ( 3 ).
Identification of U.S. Cases
CDC and New York State Department of Health (NYSDOH) Wadsworth Center conducted Oropouche virus testing for travelers who had returned from areas with known Oropouche virus circulation and had an illness that was clinically compatible with Oropouche virus disease. Clinical diagnostic testing at CDC’s Arboviral Diseases Branch and NYSDOH Wadsworth Center Arbovirus Laboratory is performed using a 90% plaque reduction neutralization test (PRNT 90 ) to detect virus-specific neutralizing antibodies in serum or cerebrospinal fluid, with titers ≥10 considered positive. CDC also conducted surveillance testing on specimens collected ≤7 days after symptom onset using an Oropouche virus real-time reverse transcription–polymerase chain reaction (RT-PCR) assay ( 6 ). This activity was reviewed by CDC, deemed not research, and was conducted consistent with applicable federal law and CDC policy.*
The Florida Department of Health (FLDOH) identified suspected cases primarily by reviewing patients who received negative test results for dengue from state and commercial laboratories and who had a clinically compatible illness and exposure to areas with potential Oropouche virus circulation. Details of epidemiologic investigations, including risk factors, clinical features, and outcomes, are captured from patient interview, clinician interview, or review of medical records using a standardized case investigation form.
Characteristics of U.S. Cases
Evidence of Oropouche virus infection was identified in 21 U.S. residents returning from travel to Cuba, including 20 in Florida and one in New York. Most patients were initially evaluated during their acute illness, but at least three patients were evaluated when their symptoms reoccurred after initial symptom resolution. The median patient age was 48 years (range = 15–94 years) and 48% were female ( Table 1 ). Pregnancy status was not included in this report for reasons of confidentiality. Reported symptoms commenced during May–July and most commonly included fever (95%), myalgia (86%), headache (76%), fatigue or malaise (62%), and arthralgia (57%). Other reported signs and symptoms included diarrhea (48%), abdominal pain (29%), nausea or vomiting (29%), rash (29%), retroorbital pain (24%), back pain (19%), and mucosal bleeding (5%) ( Table 2 ). The combination of fever and myalgia with or without other symptoms was reported in 17 (81%) patients; the combination of fever and headache was reported in 15 (71%). All three symptoms occurred in 13 (62%) patients. Overall, three were hospitalized, and no deaths were reported.
Laboratory evidence of Oropouche virus infection was identified by real-time RT-PCR in 13 patients, by PRNT 90 in seven, and by both assays in one patient. Most real time RT-PCR–positive specimens were collected on days 1–4 (median = 2.5 days; range = 1–7 days) after symptom onset. PRNT 90 –positive specimens were collected a median of 17 days (range = 9–32 days) after symptom onset.
Public Health Response
As a result of the emergence and spread of Oropouche virus in the Americas, CDC is working with state public health jurisdictions and international partners to enable rapid detection and surveillance of Oropouche virus transmission and disease to guide public health prevention measures. CDC is currently developing a plan for rapid detection and response to Oropouche virus disease cases in the United States, assisting health departments with clinical diagnostic and surveillance testing for suspected cases, working to validate a molecular assay to detect acute infections, and updating CDC’s Travelers’ Health notices † and website § on Oropouche as new information becomes available. In addition, CDC is providing clinical consultation and guidance to pregnant persons and their care providers and are tracking the impact of emerging health threats, like Oropouche virus, on pregnant persons and their infants. ¶ Although Oropouche virus disease is not nationally notifiable, CDC encourages jurisdictions to report cases voluntarily to ArboNET, the national arboviral disease surveillance system, using interim case definitions.** For questions about testing or reporting, health departments can contact [email protected] .
The 21 U.S. travel-associated Oropouche virus disease cases were all identified among U.S. residents who had traveled to Cuba. The clinical features of the travelers’ illnesses are similar to those reported in the literature ( 1 , 4 , 7 ). Most patients had a self-limited febrile illness, commonly associated with myalgia and headache with or without additional signs or symptoms, including gastrointestinal symptoms (reported by approximately two thirds of patients). At least three patients initially sought care after experiencing relapse of symptoms following resolution of the initial illness. This reported reoccurrence of symptoms is unique to Oropouche virus disease and is not typically reported in cases of similar arboviral diseases, such as dengue or Zika virus disease ( 2 ). The reoccurrence of symptoms is likely underestimated because of limitations in obtaining a complete clinical history or follow-up after the initial illness.
Among most patients, Oropouche virus disease is mild; however, two deaths in previously healthy young persons with Oropouche virus infection were recently reported in Brazil ( 3 ). In July, the Pan American Health Organization (PAHO) issued an epidemiologic alert concerning possible vertical transmission of Oropouche virus disease associated with adverse pregnancy outcomes, including fetal deaths and congenital malformations ( 3 ).
Clinicians should report suspected Oropouche virus disease cases to state, tribal, local, or territorial health departments to facilitate testing and implementation of community prevention measures and messaging. †† Information for health care providers regarding clinical features, diagnosis, and clinical management are available on CDC’s website. §§ Supportive care is recommended for clinical management of patients. Patients should be advised to avoid nonsteroidal anti-inflammatory drugs to reduce the risk for bleeding. Oropouche and dengue viruses can cocirculate and cause similar symptoms; patients with clinically suspected dengue should be managed according to dengue clinical management recommendations ¶¶ until dengue is ruled out. Interim considerations for clinical management of pregnant persons with Oropouche virus disease and infants born to these pregnant persons are available.***
Oropouche virus disease should be considered in a patient who has been in an area with documented or suspected Oropouche virus circulation ( 3 ) within 2 weeks of initial symptom onset and who experiences an abrupt onset of fever, headache, and one or more of the following: myalgia, arthralgia, photophobia, retroorbital or eye pain, or signs and symptoms of neuroinvasive disease (e.g., stiff neck, altered mental status, seizures, limb weakness, or cerebrospinal fluid pleocytosis). Because patients with Oropouche virus disease can experience reoccurrence of symptoms after resolution of the initial illness, patients might seek care >2 weeks after travel. In suspected Oropouche virus disease cases, testing should be conducted for other diseases with similar symptoms, including dengue, particularly given the recent large dengue outbreak in the Americas with approximately 11 million cases reported since late 2023 ( 8 ). Because of the concern for vertical transmission of Oropouche virus from a pregnant patient to the fetus, paired specimens should be collected from pregnant patients to confirm a recent infection.
Implications for Public Health Practice
Guidance on clinical case identification and management might be modified as the epidemiologic situation evolves, particularly if local transmission in the United States is identified and as more is learned about disease and transmission risk. Based on presently available data, the risk for sustained local transmission in the continental United States is likely low, whereas the risk for sustained transmission in Puerto Rico and U.S. Virgin Islands is unknown. CDC is working with partners to understand more about what is driving the current outbreaks and how that might affect risk of transmission. Vector competence studies are underway to understand the potential role of several U.S. Culicoides spp. of biting midges and mosquito species ( Cx. quinquefasciatus and Aedes aegypti) in Oropouche virus transmission.
Providers should advise persons of the risk for Oropouche virus disease and counsel them to use personal protective measures ††† against mosquito and biting midge bites if traveling to areas with virus circulation. Travelers should use personal protective measures for 3 weeks after return from an area with Oropouche virus circulation, or during the first week of illness in symptomatic patients to prevent further spread, especially in areas where mosquitoes or biting midges are active. Because of the risk for possible vertical transmission providers should inform persons who are pregnant and considering travel to areas with reported Oropouche virus transmission of the possible risks to the fetus. Pregnant travelers should prevent insect bites during travel §§§ and consider deferring travel to areas experiencing outbreaks of Oropouche virus disease. ¶¶¶ CDC is working with PAHO and other partners to learn more about the potential risks associated with infection with Oropouche virus during pregnancy and to increase testing capacity in the region.
Acknowledgments
Amanda Davis, Brittany Rowlette, Katelyn Wolfe, Bureau of Public Health Laboratories, Florida Department of Health; Natalia Cano, Darrell Gibson, Nicadia Gilles, Rayah Jaber, Karina Rivas, Samantha Vaccaro, Bureau of Public Health Laboratories, Florida Department of Health; Kristine Aviles, Nancy Garcia Berwick, Reyna Frias, Erica Louis Jean, Samson Marcellus, Marie France Nicolas, Alan Robles, Doris Rodriguez, Selena Singh, Amelia Pelaez Torres, Karen Velarde, Florida Department of Health in Hillsborough County; Gregory Danyluk, Bernhard Kloppenburg, Florida Department of Health in Polk County; Andres Echeverri, Michelle Persaud, Florida Department of Health in Orange County; Hollie Hall, Renee Halucha, Florida Department of Health in Lee County; Robert Singletary, St. Joseph’s Health Hospital.
Corresponding author: Carolyn V. Gould, [email protected] .
1 Bureau of Epidemiology, Florida Department of Health; 2 Bureau of Communicable Disease Control, New York State Department of Health; 3 Division of Vector-Borne Diseases, National Center for Emerging and Zoonotic Diseases, CDC; 4 Bureau of Public Health Laboratories, Florida Department of Health, Tampa, Florida; 5 Florida Department of Health in Miami-Dade County, Miami, Florida; 6 Florida Department of Health in Hillsborough County, Tampa, Florida; 7 Florida Department of Health in Polk County, Bartow, Florida; 8 Florida Department of Health in Orange County, Orlando, Florida; 9 Florida Department of Health in Lee County, Fort Myers, Florida; 10 Florida Department of Health in Sarasota County, Sarasota, Florida; 11 Arbovirus Laboratory, Wadsworth Center, New York State Department of Health, Slingerlands, New York; 12 Epidemic Intelligence Service, CDC; 13 Division of Birth Defects and Infant Disorders, National Center on Birth Defects and Developmental Disabilities, CDC; 14 Division of Disease Control and Health Protection, Florida Department of Health.
All authors have completed and submitted the International Committee of Medical Journal Editors form for disclosure of potential conflicts of interest. Andrea Morrison reports travel support for attendance at meetings from the Council of State and Territorial Epidemiologists (CSTE), the University of Kentucky–Southeastern States Occupational Network, the University of North Carolina, the American Society of Microbiology, and the Infectious Diseases Society of America. Edgar Kopp reports support for travel from the Association of Public Health Laboratories and service on the Association of Public Health Laboratories’ Biosafety and Biosecurity Committee. Joshua Lassen reports support from CSTE. Amanda M. Nichols reports travel and meeting support from the National Association of County and City Health Officials and CSTE. Alexander T. Ciota reports support from the National Institutes of Health. No other potential conflicts of interest were disclosed.
* 45 C.F.R. part 46.102(l)(2), 21 C.F.R. part 56; 42 U.S.C. Sect. 241(d); 5 U.S.C. Sect. 552a; 44 U.S.C. Sect. 3501 et seq.
† https://wwwnc.cdc.gov/travel/notices
§ https://www.cdc.gov/oropouche/about/index.html
¶ https://www.cdc.gov/set-net/about/index.html
** https://www.cdc.gov/oropouche/php/reporting/index.html
†† https://emergency.cdc.gov/han/2024/han00515.asp
§§ https://www.cdc.gov/oropouche/hcp/clinical-overview/index.html
¶¶ https://www.cdc.gov/dengue/hcp/clinical-care/index.html
*** https://www.cdc.gov/oropouche/hcp/clinical-care-pregnancy/index.html ; https://cdc.gov/oropouche/hcp/clinical-care/infants.html
††† https://www.cdc.gov/oropouche/prevention/index.html
§§§ https://www.cdc.gov/mosquitoes/prevention/preventing-mosquito-bites-while-traveling.html
¶¶¶ https://wwwnc.cdc.gov/travel/notices/level2/oropouche-cuba
- Pinheiro FP, Travassos da Rosa AP, Vasconcelos PFC. Oropouche Fever [Section 17]. In: Feigin RD, Cherry JD, Demmler GJ, Kaplan SL, eds. Textbook of pediatric infectious diseases. 5th ed. Philadelphia, PA: Saunders; 2004:2418–23.
- Azevedo RS, Nunes MR, Chiang JO, et al. Reemergence of Oropouche fever, northern Brazil. Emerg Infect Dis 2007;13:912–5. https://doi.org/10.3201/eid1306.061114 PMID:17553235
- Pan American Health Organization, World Health Organization. Epidemiological alert: Oropouche in the Region of the Americas – 1 August 2024. Washington, DC: Pan American Health Organization; Geneva, Switzerland: World Health Organization; 2024. https://www.paho.org/en/documents/epidemiological-alert-oropouche-region-americas-1-august-2024
- Durango-Chavez HV, Toro-Huamanchumo CJ, Silva-Caso W, et al. Oropouche virus infection in patients with acute febrile syndrome: is a predictive model based solely on signs and symptoms useful? PLoS One 2022;17:e0270294. https://doi.org/10.1371/journal.pone.0270294 PMID:35881626
- European Centre for Disease Prevention and Control. Threat assessment brief: Oropouche virus disease cases imported to the European Union. Stockholm, Sweden: European Centre for Disease Prevention and Control, 2024. https://www.ecdc.europa.eu/en/publications-data/threat-assessment-brief-oropouche-virus-disease-cases-imported-european-union
- Naveca FG, Nascimento VAD, Souza VC, Nunes BTD, Rodrigues DSG, Vasconcelos PFDC. Multiplexed reverse transcription real-time polymerase chain reaction for simultaneous detection of Mayaro, Oropouche, and Oropouche-like viruses. Mem Inst Oswaldo Cruz 2017;112:510–3. https://doi.org/10.1590/0074-02760160062 PMID:28591313
- Castilletti C, Mori A, Matucci A, et al. Oropouche fever cases diagnosed in Italy in two epidemiologically non-related travellers from Cuba, late May to early June 2024. Euro Surveill 2024;29:2400362. https://doi.org/10.2807/1560-7917.ES.2024.29.26.2400362 PMID:38940002
- Pan American Health Organization, World Health Organization. PLISA health information for the Americas: dengue. Washington, DC: Pan American Health Organization; Geneva, Switzerland: World Health Organization. Accessed August 14, 2024. https://www3.paho.org/data/index.php/en/mnu-topics/indicadores-dengue-en.html
* Within cells, X = sign or symptom reported; dash = no sign or symptom reported.
Suggested citation for this article: Morrison A, White JL, Hughes HR, et al. Oropouche Virus Disease Among U.S. Travelers — United States, 2024. MMWR Morb Mortal Wkly Rep. ePub: 27 August 2024. DOI: http://dx.doi.org/10.15585/mmwr.mm7335e1 .
MMWR and Morbidity and Mortality Weekly Report are service marks of the U.S. Department of Health and Human Services. Use of trade names and commercial sources is for identification only and does not imply endorsement by the U.S. Department of Health and Human Services. References to non-CDC sites on the Internet are provided as a service to MMWR readers and do not constitute or imply endorsement of these organizations or their programs by CDC or the U.S. Department of Health and Human Services. CDC is not responsible for the content of pages found at these sites. URL addresses listed in MMWR were current as of the date of publication.
All HTML versions of MMWR articles are generated from final proofs through an automated process. This conversion might result in character translation or format errors in the HTML version. Users are referred to the electronic PDF version ( https://www.cdc.gov/mmwr ) and/or the original MMWR paper copy for printable versions of official text, figures, and tables.

COMMENTS
Footnotes. Current or past transmission is defined as having had locally acquired, mosquito-borne Zika cases. Some countries with temporally and geographically limited Zika virus transmission in the past may be classified as having no reported cases if they meet the following criteria: 1) had no confirmed locally acquired Zika virus disease cases for 12 months; 2) are located in a subtropic or ...
Zika is spread mostly by the bite of an infected Aedes species mosquito ( Ae. aegypti and Ae. albopictus ). Zika can be passed from a pregnant woman to her fetus. Infection during pregnancy can cause certain birth defects. Your decision to delay or cancel travel is personal and complex.
"One of the main reasons not to travel to areas at risk of Zika is that it is known to cause birth defects in babies born to parents infected with the virus," Dr. Goje notes.
Your decision to travel or live abroad is personal and complex. The large Zika virus outbreaks that occurred during 2015 and 2016 in the Americas are over, but Zika virus continues to be a risk in many countries around the world. Review CDC's latest Zika recommendations for travelers and people living abroad.
Homepage for CDC's Zika virus website. Skip directly to site content Skip directly to search. An official website of the United States government ... About Symptoms and Complications How It Spreads Testing Prevention Treatment Travel and Living Abroad Areas at Risk for Zika View All Zika Virus. Preventing Zika. Learn how to prevent Zika by ...
Zika epidemiology update (February 2022) History of Zika virus; Symptoms. Most people infected with Zika virus do not develop symptoms. Among those who do, they typically start 3-14 days after infection, are generally mild including rash, fever, conjunctivitis, muscle and joint pain, malaise and headache, and usually last for 2-7 days ...
Zika transmission persists in several countries but has generally been at low levels throughout 2018 to the present. Since the last epidemiological update in 2019, two countries have been added to the list of countries with evidence of autochthonous, mosquito-borne transmission, based on peer-reviewed published data. Findings underscore the ...
Since 2017, the number of reported Zika virus disease cases has declined worldwide, but occasional increases in cases have been noted from some countries. In 2020, only 4 Zika virus cases were reported in US international travelers. See current information on Zika virus transmission and travel guidance. Clinical Presentation
Zika virus (ZIKV) disease is a mosquito-borne disease caused by Orthoflavivirus zikaense. The disease is most frequently an acute febrile illness with myalgia, skin rash, arthralgia, and neurological signs. Sub-clinical and asymptomatic cases are common. ZIKV was first recognised in Uganda in 1947 and sero-epidemiological evidence suggests wide ...
The most recent Zika virus outbreak in the United States (U.S.) occurred in 2016, and 5,168 people were diagnosed with Zika that year. Since 2018, Zika cases have been on the decline, and as of July 2022, there are no current outbreaks of Zika worldwide. This article will highlight important facts and statistics about the Zika virus.
The Zika virus dominated headlines in 2016 when some infected women had babies with significant birth defects. At the time, the threat of the mosquito-borne virus caused major concern, leading many travelers to change their travel plans. Since Zika is no longer front-page news, people might think the virus is no longer a threat.
To find out if your destination is a country or area with risk of Zika virus, consult the Travel Advice and Advisories page, and select your destination. Information on diseases spread by insects, such as Zika virus, is found under the 'Health' tab. Zika virus. Zika virus typically causes mild illness lasting only a few days. Many people ...
Distribution of travel-associated Zika virus disease cases reported to ECDC, by place of infection, 2022. ... the data presented only cover the years 2018−2022 and therefore do not imply ongoing transmission. Data from 2023 are currently being validated and will be made available by the end of 2024.
Zika virus may be one step away from explosive outbreak. 12 April 2022. Michelle Roberts. Digital health editor. EPA. The virus has been linked to cases of microcephaly. A new outbreak of Zika ...
Zika-Free Caribbean Islands. The worst thing that can happen while traveling abroad is getting sick. Every year, it seems a new virus is emerging, and since 2016, we are 'blessed' with the Zika Virus. This nasty virus is spreading to remote and gorgeous Caribbean islands. The Centers for Disease Control and Prevention (CDC) keeps an up to ...
Zika Virus in Pregnancy. A pregnant woman can pass Zika virus to her fetus. Infection during pregnancy can cause serious birth defects. CDC recommends special precautions for the following groups: Women who are pregnant: Should not travel to any area of Brazil below 6,500 feet. If you must travel to one of these areas, talk to your doctor first ...
Background. Prior to 2014, very few travel-associated cases of Zika were identified in the United States. In 2015 and 2016, large outbreaks of Zika virus occurred in the Americas, resulting in an increase in travel-associated cases in U.S. states, widespread transmission in Puerto Rico and the U.S. Virgin Islands, and limited local transmission in Florida and Texas.
Contact FDA. General Info/Consumers 1-888-INFO-FDA / (1-888-463-6332) Report a Fraudulent Zika Product Report form and instructions. Press Office of Media Affairs [email protected] 301-796-4540 ...
Microcephaly is far less prevalent, but it, too, is still occurring: Brazil saw 163 cases of Zika-linked microcephaly in 2022, according to the Pan American Health Organization, down from 2,033 in ...
The state reported 27 cases of Zika virus infection in 2021, followed by three cases in 2022, and 15 cases in 2023. Subscribe to Outbreak News TV on YouTube Zika is a disease caused by infection with Zika virus and typically occurs in tropical and subtropical areas in parts of Africa, the Americas, Asia, and the Western Pacific.
Zika is a virus spread by Aedes mosquitoes (Ae. aegypti and Ae. albopictus), sexual contact, or the birth process, that has no vaccine or treatment.Although usually harmless, Zika can on rare occasions cause serious health issues, such as swelling of the brain, blood disorders, or Guillain-Barré syndrome.Zika is a particular concern for pregnant women, as contracting the disease during ...
Most Zika virus infections are spread through the bite of an infected Aedes species of mosquito. The primary mosquito that carries the disease, Aedes aegypti, is not established in Pennsylvania.Another type of mosquito, Aedes albopictus, is able to carry Zika virus, although it is less efficient at spreading viruses like Zika because they feed on both people and animals.
At the start of the Zika virus (ZIKV) epidemic in 2015, ZIKV spread across South and Central America, and reached parts of the southern United States placing pregnant women at risk for fetal ...
A little-known disease spread by insect bites has turned deadly, and health officials are sounding the alarm. More than 8,000 cases of Oropouche virus have been reported this year as of August 1.
"This mode of transmission makes it similar to other arboviruses like dengue and Zika." One of the significant factors contributing to its rapid spread in Europe and the US is increased travel and global interconnectedness, he states, which facilitates the movement of infected individuals and vectors across borders. Dr Srinivasan echoes this.
Committee: House Oversight and Accountability: Related Items: Data will display when it becomes available. Date:
More than 20 people returning to the U.S. from Cuba have been infected with a virus transmitted by bugs in recent months, federal health officials said Tuesday. They all had Oropouche virus ...
The 21 U.S. travel-associated Oropouche virus disease cases were all identified among U.S. residents who had traveled to Cuba. The clinical features of the travelers' illnesses are similar to those reported in the literature (1,4,7). Most patients had a self-limited febrile illness, commonly associated with myalgia and headache with or ...